1. Introduction
Because feed makes up the largest portion of the cost of production (between 37% and 70%), which is caused by the high cost of raw materials, it is regarded as one of the crucial factors that must be considered in fish farming activities (both intensive and semi-intensive)1. Macrophytic plants cover the surface of ponds and grow very quickly; in order to manage their high output, they are frequently harvested and utilized as cattle fodder2. The most frequent issues that prevent their use as ingredients in fish feed include macrotrophic amino acid imbalance, the presence of anti-nutrients, low digestibility, and poor palatability3. Therefore, its nutritional value can be improved and their inclusion in fish diets can be increased through the fermentation process, as it is a simple and inexpensive process, and there can be an increase in nutrients through feed synthesis 4)(5) . The fermentation process has been shown to reduce nutrient-inhibiting substances as well as fiber6.
Hornwort or coontail (Ceratophyllum demersum) is a perennial aquatic weed, 20-100 cm length, with simple, branched leaves belonging to the Ceratophyllaceae family. This plant is widespread in most of the southern regions of Iraq, especially the marshes south to Abu Al-Khaseeb7. With regard to its economic importance, the hornwort species are considered a shelter and food source for fish, especially small ones8. It is also used as an excellent cover for newly hatched fish larvae after cutting and spreading them in the aquarium9.
One of the biggest members of the Cyprinidae family is the grass carp (Ctenopharyngodon idella Val.), which is the only species in the genus10. Freshwater grass carp are herbivorous fish that prefer slow-moving or still bodies of water with lots of vegetation. Filamentous algae and floating or partially submerged plants with brittle or delicate tissues are desired. Generally speaking, grass carp were seen utilizing a high rate of body weight to consume local macrophytic like Ceratophyllum demersum, Myriophyllum verticillatum, Phragmites australis, Typha domingensis, and Juncus rigidus11. The purpose of the research, and according to the proposed experiments, is to evaluate the effect of both raw and fermented hornwort plant on growth performance, feed efficiency, digestibility and carcass composition of young grass carp compared to a control diet without it.
2. Materials and methods
2.1 Plant collection
The hornwort plant was collected from the Gharat River (Qalat Sakar, 100 km north of Dhi-Qar governorate, southern Iraq). To get rid of stuck-on dirt, the plant was carefully rinsed with tap water, chopped into small pieces (1-2 cm), dried by the sun for two days until the water content was dropped to about 50%, then divided into two parts, one incorporated in the diets as raw (dried by oven at 60 °C for 48 hours finely ground), while the other was fermented.
2.2 Fermentation process
According to El-Sayed12, the fermentation was carried out by adding 5% molasses and 2 ml of acetic acid13 per kilogram of hornwort, mixing continuously, and storing for 50 days in a room at 35 ± 2.2 °C. Then, to encourage decomposition, the containers were routinely flipped over every week. The fermented plant was then dried in an oven at 60 °C for 48 hours; finely ground, packaged, and kept in the fridge until needed.
2.3 Diets formulation
Three test diets were formulated by using locally available feeding ingredients and adding either raw (HR) or fermented (HF) hornwort (Table 1) at a 20% level to compare with the control (C) diet. The experimental diets were formulated to provide 40% crude protein, and approximately 410 Kcal 100-1 g gross energy by adding 20% of each of the alternative ingredients separately to the control diet to completely compensate for barley, a portion of wheat bran, and 20% of fish meal.
2.4 Growth and feed efficiency
Forty-five young grass carp (4.03±0.16 g) were collected from Marine Science Center fish ponds, transported to the laboratory, randomly distributed into nine plastic aquariums (22-liter capacity) at a stocking density of five fish per tank, acclimatized, and fed with the experimental diets for a period of two weeks. Each of the trial diets was fed to triplicate groups of fish at 3% of their body weight twice a day, at 9:00 am and 4:00 pm, for 12 weeks. Water quality parameters were checked periodically (Table 2). Fish weights were recorded every four week intervals at the end of the experiment. Fish weight and the amount of feed consumed in each tank were recorded to calculate the following growth indices:
Weight gain (WG) = fw (g) - iw (g)
Specific growth rate (SGR) % day-1 = ((ln final weight (g) - ln initial weight (g) ×) /days of rearing) × 100
Feed conversion ratio (FCR) = food consumed (g)/ weight gained (g)
Protein efficiency ratio (PER) = weight gained (g)/ protein consumed (g)
Protein productive value (PPV) % = (protein gained (g)/ protein consumed (g)) × 100
Survival rate (SR) % = initial number of fish stocked mortality / initial number of fish stocked × 100
2.5 Satiation level
The trial of satiation lasted for ten days, after the digestibility trial, during which both groups of fish were fed to excessed amount of feed as one meal daily, for an hour (8:00 to 9:00 am), uneaten feed was collected by siphoning, dried by air and weighed. Satiation level was estimated as a percentage of body weight according to the following equation:
-Satiation level (%) = food consumed (g)/body weight (g) × 100
2.6 Evacuation time and rate
The length of time between feeding and the cessation of feces production was measured using diets that had been stained with two distinct colours: red (carmine 1%) and green (chromic oxide 1%). The following calculation was used to calculate the evacuation rate: -
-Evacuation rate (g food h-1) = food consumed (g)/ evacuation time (min)/ 60
2.7 Digestibility
The apparent digestibility coefficients (ADC) of the diets were conducted according to Felix and Brindo14, by incorporation of 1% chromic oxides indicator in each diet. Fishes were acclimated to the experimental diets during the first couple of days and no feces were collected. The experiment lasted for three weeks. Diets were given daily at 9:00 am, and one hour after food consumption uneaten feed and feces were removed by siphoning. Feces collected from triplicate treatments on 20-min intervals were pooled, dried by air and stored for further analysis. The amount of chromic oxide present in the feeds and fecal samples was estimated by digestion with concentrated nitric and perchloric acids, and the absorption was measured in the atomic absorption at 357.9 nm. The ADCs were calculated according following equations:
Total apparent digestibility (ADS total) % = 100 - (100 × (% indicator in food/ % indicator in feces))
Nutrient apparent digestibility % = 100 - (100× (% indicator in food/ % indicator in feces) × (% nutrient in feces/ % nutrient in food))
2.8 Chemical composition
Physio-chemical methods were used according to Porto and others15 to determine the chemical composition of fish, feces, and feed ingredients. In an oven with a 105 °C setting, weight loss was used to measure moisture. Ash was produced by burning a known quantity of the sample at 550 °C to a constant weight. Crude protein was calculated by converting total nitrogen, which was measured using the Kjeldahl method, into protein. The 6.25 factor was applied. Using the Soxhlet procedure, petroleum ether was used to extract the total lipids. The total calorie content was determined using the equivalent caloric values for proteins, lipids, and carbohydrates, which were 5.5, 9.1, and 4.1 kcal/g, respectively. Carbohydrates were estimated using the following equation:
Carbohydrates = 100 - (moisture % + protein% + lipid% + ash%)
2.9 Statistical analysis
The data were displayed as mean ± SD. To determine the impact of dietary treatment on fish performance, the data were subjected to a one-way analysis of variance (ANOVA). Data were analysed using the IBM SPSS application, Version 26 2013. LSD's multiple range tests were used to compare mean differences at the P<0.05 level.
3. Results
The chemical composition of hornwort meal is shown in Table 3 for both the (HR) and (HF) kinds. It can be seen that while the amount of crude protein decreased during the fermentation process, the amount of lipids, particularly carbohydrates, dramatically rose. In terms of ash content, the HR had a high level.
Table 3: Chemical composition of raw and fermented hornwort meal
| (HR) | (HF) | |
| Moisture | 1.19±0.2 | 1.38±0.09 |
| Crude protein | 18.21±0.22 | 16.62±0.69 |
| Lipids | 2.53±0.55 | 3.65±0.53 |
| Carbohydrates | 43.95±0.63 | 53.00±1.45 |
| Ash | 34.11±0.16 | 25.35±0.13 |
Table 4 shows the growth performance of young grass carp. The addition of hornwort meal to the diet, whether HR or HF, had a negative effect because the control group (C) was superior in a significant (P>0.05) manner. In the same way, there was a significant worsening (P>0.05) beyond the control group (C) in terms of feeding efficiency (FCR, PER, and PPV) in the RH and FH. All groups achieved the same survival rates (SR).
Table 4: Survival, growth and feed efficiency related parameters of young grass carp fed with experimental diets

Data shown as mean±SD of three replicates. Means within the same row that share a common letter do not differ statistically (P<0.05).* initial weight; ** final weight.
The results in Table 5 exhibit the satiation level in young grass carp, indicating an increase in RH and HF over the control group (C) (P<0.05). It is noteworthy that digestible energy increased in the control group (C). On the other side, it should be mentioned that HR had the longest evacuation time (21:54 hh:mm). Consequently, the statistical analysis demonstrated that this experimental group (P<0.05) slows the control (C) in terms of evacuation rate.
Table 5: Satiation level, digestible energy, evacuation time and evacuation rate of experimental diets fed to young grass carp during experiment

Data shown as mean±SD of three replicates. Means within the same row that share a common letter do not differ statistically (P<0.05).
The ADC total and the nutritional digestibility values for young grass carp are shown in Table 6. ADCtotal in the control group (C) was high (P<0.05). When it came to the ADClipid, the addition of RH and FH foods increased the effectiveness of digestion, whereas the statistical analysis revealed that there were no appreciable variations (P>0.05) in the ADCprotein. Contrarily, the ADCcarbohydrate was a little bit different, with the RH recording the lowest value (47.50%) and a significant difference between the raw treatment and the control treatment (C). There were no discernible changes (P>0.05) in ADCAsh across all the groups.
Table 6: Nutrient digestibility (%) of experimental diets fed to young grass carp
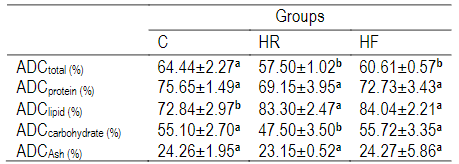
Data shown as mean±SD of three replicates. Means within the same row that share a common letter do not differ statistically (P<0.05).
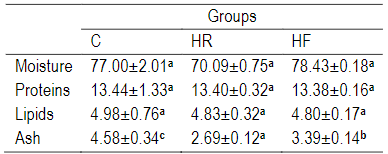
The chemical analysis of the fish bodies at the end of the experiment is shown in Table 7. There were no significant differences (P>0.05) in each of the moisture, protein and fat, while the ash treated with RH had a low value and a significant difference (P<0.05) from the others.
4. Discussion
Fermentation improves the nutritional value of forage materials by reducing the effect of anti-nutrients as well as reducing fiber4. Generally, macrophytics are challenging to ferment, and little is known about their fermentative abilities6. The present results show a decrease in the protein content in the fermented macrophytic compared to what was originally present in the raw; it is known that the fermentation process depends strongly on the type of plant, perhaps due to the addition of molasses (protein 5%, carbohydrates 50%), which lead to a higher percentage of carbohydrates at the expense of the rate of protein or the decrease in protein in the fermented treats, may be due to the metabolic activity of Lactobacillus sp. to balance the ratio of nitrogen to carbon16. However, these results agree with several studies, including the studies of El-Sayed12 and Cruz and others17, on the water hyacinth (Eichhornia crassipes) and the Cachama blanca (Piaractus brachypomus), respectively; also, the studies of Ilias and others18 on fermented seaweed, and Bairagi and others 4) on duckweed (Lemna polyrhiza).
The SGR of fish fed on RH-containing diet decreased significantly (P>0.05) in comparison to the control (C), and this could be because of the high fiber content of the diet, which is estimated to account for 15% of its chemical composition19, or maybe it contained anti-nutrients such as oxalic acid20, or due to the low digestibility of the dry matter21, and this is consistent with the present results that indicate that it has a lower ADCtotal with a significant difference (P<0.05) for all other groups except HF.
On the other hand, the results noted that the FCR was higher in the fish fed on diets containing RH, and this was accompanied by a decrease in growth rates compared to the fish fed on the control (C). These results are in line with those of Laining and others22, who studied rabbitfish (Siganus guttatus) and confirmed that the amount of hornwort (Ceratophyllum sp.) in the diet increased the FCR value. Moreover, they attributed this to the presence of fibers like lignin and cellulose, which are known to be present in aquatic plants23. However, the increase in fish food consumption may be the cause of this, as fish fed on diets containing RH have the highest satiation level (2.77% of body weight). NRC24 stated that high FCR means that the nutrients in the diet are not used optimally, as evident in the low ADC by the fish in this group, and this is consistent with what AbdelTawwab25 found of high FCR (4.2) in Tilapia zillii fed on sun-dried fern (Azolla pinnata) by 25% compared to the control diet. Moreover, the decreases in PER in fish fed both on HR and HF may be due to the presence of anti-nutritive products that limit protein utilization26.
Table 5 shows that fish fed on HR had the highest level (P<0.05) of satiation (2.77%), which may be related to the fact that their ADCcarbohydrate had decreased (47.50%), and that they needed to compensate for the lack of digested energy26. However, Al-Tamimi27 indicated that an increase in the ratio of P:E in the diets was accompanied by a decrease in the level of satiation, and this is consistent with the present study (Table 2), as the value of P:E in the control (C) was 98.5 mg protein kilocalorie-1, which recorded the lowest level of satiation (2.31%).
The present results are showing that the evacuation time was long in the HR. However, due to the size of the meal, because the percentage of food ingested by the fish fed on this diet was greater than the other diets, as the satiation level reached (2.77%), it led to a higher rate of food emptying for the fish of this diet, and the evacuation rate is associated with a direct relationship with the size of the meal28.
The most crucial factors in feed formulation are the nutrients' quantity, quality, and digestibility, especially the nutrients' digestible protein and energy 29)(30) . The apparent digestibility coefficients (ADC) offer useful information for the creation of nutrient-dense diets that are also practical from an economic standpoint4. Given that Adewumi31 noted that a diet containing a high percentage of fiber can result in a decrease in digestibility, and considering the high percentage of fiber in hornwort, estimated to make up about 15% of its chemical composition, these findings suggest the cause of the low ADC in the HR and HF groups18. In addition, Cruz and others32 suggested that the high percentage of ash in aquatic plants may impair the digestibility of diets, further reducing growth. Lall33 made the observation that the quantity of anti-nutrients in various nutrients may have an impact on how easily they are absorbed. Aquatic plants typically exhibit poor palatability and digestibility; however, non-traditional feed ingredients frequently have a low digestibility 34)(35) .
HR and HF recorded the highest values for ADClipid significantly (P<0.05), this may be due to an attempt to compensate for the lack of digested energy as a result of the low digestibility of other energy sources (protein and carbohydrates). Gao and others36 indicated the ability of grass carp to exploit fat as an energy source in a better way compared to carbohydrates. On the other hand, Abimorad and Carneiro37 found similar results with pacu (Piaractus mesopotamicus), where they showed the ability of this fish to use fats for energy and store protein for growth.
As hard-to-digest fibers enclose digestible substances like protein and carbohydrates and shield them from the action of digestive enzymes on them, Hepher38 contends that a high content of fiber in diets causes a decrease in digestibility. This results in a decrease in digestibility in fish, which explains why ADCCarbohydrate decreased in HR, which amounted to 47.50% (Table 6). Results showed that the amount of digestible energy in the diets had an impact on satiation level. As dietary digestible energy decreased, satiety level rose.
The present results showed that the fermentation of macrophytic did not affect the chemical composition of the fish body in general, and this is consistent with the study of Cruz and others17 and the study of El-Sayed12, who reported that the fermentation of the water hyacinth did not affect the chemical composition of the body of the Nile tilapia fish. The chemical composition of the fish body varies greatly according to a number of factors, the most important of which are the type of fish, size, sexual status, the season of feeding, and the natural activity39. It is known that the growth and chemical composition of the fish's body can be affected by dietary diversity, and that the content of the fish's body moisture, protein and fat varies according to the type of diet fed to the fish40.
5. Conclusions
These findings imply that although aquatic plants have a promising future in aquaculture, hornwort had an adverse effect on growth performance and feeding efficiency, which makes it unsuitable in aquatic feed. In general, this plant may need further studies and reconsideration of the methods used.