1. Introduction
Replacement heifers in dairy systems represent the second cost after feeding of dairy cows and approximately 13-25% of total farm expenses1)(2. The raising period in heifers is extended from birth to first calving, and it is considered one of the most important periods in the life of a dairy cow. In the last two decades, many studies reported that an adequate heifer rearing can have short- and long-term effects on health, welfare, and production3.
The target age at first calving and the body weight (BW) for Holstein cows are between 22 to 24 mo-old and 82% of their mature BW (after calving), respectively, for which heifers must conceive at around 15 mo-old with 60% of their mature BW4. Achieving those goals ensure minimizing the non-productive periods and the cost of rearing in the dairy farm5. For that purpose, management practices during heifers rearing should allow an adequate body growth and reproductive development. In this regard, puberty must be achieved several months before first breeding, to maximize heifer´s fertility at first conception6. In addition, monitoring the body and skeletal development through different measurements -i.e. BW, average daily gains (ADG), withers height (WH), heart girth (HG), and body condition score (BCS)- during the rearing of the heifer is fundamental to achieve those targets7.
Several factors are known to affect the body and reproductive development in dairy heifers. In this review, we will focus on two main factors: feeding level and social environment. An adequate feeding level in the pre- and post-weaning phases has been shown to increase growth rates, and decrease disease susceptibility and mortality, with long-term effects such as an increased milk production8. In turn, social environment in heifers reared in groups, and the effects of management practices on social structure of the group are known to affect the energy metabolism, feeding behavior and body and reproductive development9)(10. Underlying mechanisms involved in the differences in body and reproductive development according to feeding level and social environment have been linked to feed intake, feeding behavior and metabolic status of the heifers.
In Uruguay, published information focusing on the management of the dairy heifer during the rearing period is scarce. The average age at weaning and first calving is 75 days11 and 30 mo-old, respectively, while almost 35% of heifers still calve with more than 30 mo-old12. In particular, age at first calving is considerably higher than recommended targets. Little is known regarding the management of dairy heifers after weaning. A recent study surveyed the management carried out in 13 contract-rearing farms in Uruguay during 201813, which reared almost 12,500 heifers from 372 producers in 19,816 ha. Of the total area, 53% was occupied by range fields, and almost 30% by sown permanent grasslands and annual crops for grazing. On average, Holstein heifers entered the farm with 160 kg and left with 495 kg (approximately 2 months before calving), with an average stay of 23 months, which yields an ADG of 476 g/d during the stay. The ADG is 14% higher than that reported in a similar survey carried out 10 years earlier on the same set of contract-rearing farms14, which, although it suggests a more refined management of feeding, is still insufficient to achieve the previously mentioned targets for heifer growth.
The aim of this paper is to briefly review the main factors affecting the onset of puberty. Specifically, we will discuss the results of studies performed in Uruguay that evaluated the effects of feeding level during the pre- and post-weaning period, and social environment -social dominance and social regrouping- during the post weaning period on body development, metabolic status, and onset of puberty in dairy heifers.
2. Puberty, body development and metabolic status in dairy heifers
Optimum heifers rearing can be defined as the one that allows to maximize milk production at first lactation with an adequate age and minimum costs5. As previously mentioned, it has been reported that heifers must conceive at 15 mo-old, for which the time of onset of puberty becomes a determining factor, especially in seasonal calving systems. Puberty in females’ mammals is defined as the first ovulation accompanied with estrus15. An early study demonstrated that pregnancy rates of heifers bred in the third estrus increased 21% compared to when heifers are bred in the puberal estrus6; thus to maximize fertility it is important that heifers begin puberty several months before first service.
At the ovarian level, female calves have growing follicles long before the onset of estrous activity16. In 2-week-old calves, there is already an increase in follicle-stimulating hormone (FSH) concentrations, which initiates the emergence of the first waves of follicular development, with a behavior like that of an adult animal, but without the occurrence of ovulation17. In the prepubertal stage, estradiol inhibits luteinizing hormone (LH) secretion due to the negative feedback it exerts at the hypothalamic-pituitary level, which causes the regression of the dominant follicle of the wave18.
Prior to the onset of puberty, both the amplitude and frequency of LH pulses from the pituitary gland increase, as the hypothalamic-pituitary axis becomes less sensitive to the negative feedback control of estradiol. This change in the regulation of LH secretion by estradiol begins approximately 50 d before puberty in heifers18. Ultimately, this increase in LH pulsatility stimulates follicular development and estradiol secretion, which trigger the preovulatory LH surge and subsequent ovulation of the dominant follicle19.
Dairy heifers are expected to reach puberty with a certain BW relative to mature BW20)(21. For example, it has been suggested that heifers of dairy or dual-purpose breeds (e.g. Holstein, Jersey, Brown Swiss) reach puberty at a younger age and at a lower BW relative to adult BW (around 55% of adult BW) compared to heifers of beef breeds (egg Angus, Hereford, Charolais) or Bos indicus (e.g. Brahman, Nellore), where puberty is reached when the animals achieve 60 or 65% of adult BW, respectively22. Typically, puberty in heifers occurs at 11 months of age, but there is a considerable range in this trait, with dairy heifers attaining puberty as early as 8 months, and Bos indicus heifers attaining puberty at 24 months or more16.
However, the relationship between the time at which puberty is reached and the BW of the heifer is not clear. It has been observed that the BW at which menarche is reached varies less than the age at which it occurs, which leads to the theory that it is necessary to achieve a critical BW to trigger the endocrine events that lead to puberty23. Something similar occurs in rats, where puberty occurs once a critical BW is reached24. Although this theory was extrapolated and supported by information obtained with heifers of beef25)(26)(27 and dairy breeds28)(29)(30, other studies reported that heifers maintained under different feeding plans reached puberty with different BW16)(31)(32.
Because indirect markers of the amount of body fat were less variable than the BW with which females reached menarche, it was suggested that it is necessary to reach a minimum amount of body fat capable of inducing the endocrine changes that trigger puberty33. While some studies could not prove the theory that heifers reach puberty by reaching a minimum amount of fat34)(35, in others no differences in body composition were observed at puberty in heifers subjected to different feeding plans and that reached puberty at different ages30, which would support this theory.
Body and reproductive development in heifers are influenced by the somatotropic axis, which is integrated by the growth hormone (GH), the GHRH (GH-releasing hormone), and the insulin-like growth factor 1 (IGF-1)36. The GH is synthesized and secreted by the somatotrophs of the anterior pituitary gland, and growth actions are thought to be mediated indirectly through the generation of IGF-137. The latter enhances cellular proliferation, differentiation, and maturation of many tissues, including bone, cartilage, and skeletal muscle38. Both IGF-I and GH act directly at the pituitary to inhibit GH secretion and indirectly at the hypothalamus by suppressing GHRH release39. At the adipose tissue, GH stimulates lipolysis and reduces the lipogenic response to insulin, while IGF-1 stimulates lipogenesis40. In addition, IGF-1 has important effects on development and reproductive performance in heifers: high IGF-1 levels act as a metabolic signal to the hypothalamus and stimulates gonadotropin secretion38. Moreover, there are complex feedback mechanisms between the somatotropic axis and some metabolic hormones, especially insulin and glucose, which influence body development and reproduction. Insulin is an important regulator of lipogenesis and IGF-1 actions39; while glucose is the main energy source for ovarian function41)(42. In turn, both insulin and IGF-1 exert direct effects on follicular cell proliferation and steroidogenesis in vitro43, and their increase is positively associated with whether they ovulate and with in vivo follicular growth39. In the case of leptin, which is mainly produced by the adipose tissue, it would fulfill a permissive role in the establishment of puberty, informing the reproductive axis about the energetic state of the animal; however, it is less sensitive to the changes associated with the level of nutrition when the percentage of body fat exceeds a threshold, which would reaffirm the notion that a minimum body reserves are required to reach puberty44.
An increase in the concentration of IGF-1 and insulin is positively associated with a lower age at first heat in heifers41. Higher insulin and IGF-1 concentrations are associated with increases in pulsatile LH secretion45. In contrast, low concentrations of IGF-1 would determine a delay in the age of puberty due to a decrease in the synthesis of oestradiol at the ovarian level, that would prevent the necessary stimulus for the preovulatory LH surge to occur38. In particular, the prepubertal increase in IGF-1 concentrations may act as a modulator of the onset of puberty46)(47. The increase in insulin secretion acts directly or indirectly through glucose uptake by cells, altering different processes involved in the initiation of cyclicity48)(49. In this sense, adequate glucose levels appear to be necessary for insulin to have any effect on LH concentrations50.
Collectively, the changes in the concentrations of the mentioned metabolic hormones would promote changes at the neuroendocrine system level. For example, at the level of the hypothalamus they promote a reduction in the abundance of neuropeptide Y mRNA (which has a negative action at the level of neurons of GnRH) and an increase in the abundance of proopiomelanocortin mRNA (which has a positive action on kisspeptin neurons), which in turn stimulates gonadotropin secretion. These modifications reduce the sensitivity to negative feedback of estradiol on GnRH secretion, which increases the pulsatile secretion of GnRH and LH and stimulates the follicular growth that determines the first ovulation51.
Increasing BW without the corresponding skeletal development has been linked to calving problems, impaired mammary gland development and lower milk production at first lactation52. In addition, fat deposition in dairy heifers has been linked to mammary gland development rather than ADG53. Body condition represents a measure of the fat deposition of the animal, for which there is a scale designed for dairy heifers54. However, withers height and width of the hip would also be appropriate parameters since they are not affected by the BCS of the animal7. In this sense, the physiological state of the female, especially in relation to the age of puberty, would be a major regulator of mammary development55. From 3 months of age to puberty, the growth of the mammary gland is proportionally greater than the body growth of the animal (i.e. allometric growth period), which is especially associated with the increase in the deposition of adipose tissue. A disturbance in the mammary gland growth at this stage affects the potential for future milk production: for example, an important increase in the nutritional level before puberty affects the secretion of lactogenic complex hormones and reduces the growth of the mammary parenchyma29. Therefore, a correct body and mammary gland development are highly influenced by management during the prepubertal period, and are determinant of the productive future of the animal.
3. Effect of feeding level during different periods on pubertal development
Since many decades ago, nutrition is known to be one of the main factors that affects attainment of puberty in female cattle. It is generally reported that a greater rate of body weight during the post-weaning period, thus, a greater plane of nutrition, has been systematically associated with a lower age at puberty, both in dairy56)(57)(58 and beef31)(59)(60) heifers. In Uruguay, this effect was reported in beef heifers maintained on a greater plane of nutrition before61 and after62 weaning. Chronic nutrient supply restriction determines a delay on puberty onset63, while an acute feed restriction may determine that heifers that were already cycling become anovulatory64)(65.
It is accepted that the effects of an improvement in the postnatal feeding plane on an earlier onset of puberty are generally related to an earlier escape of the hypothalamus-pituitary axis from the negative oestradiol feedback on pulsatile secretion of LH16. Although the exact mechanisms that link nutrition with the onset of puberty are not yet clear, they involve changes at the level of different molecules that provide information at the hypothalamus level about the balance of nutrients in the animal, associated with fluctuations in weight and/or level of body reserves66. For example, an improvement in the postnatal nutritional level of heifers increases the circulating concentrations of leptin, IGF-I and insulin, and metabolites like glucose, which in turn have been associated with a faster onset of puberty46)(47)(56)(67)(68)(69. From an evolutionary point of view, it implies that the female must reach a threshold amount of accumulated energy before the reproductive processes that lead to a successful gestation are activated15.
In dairy cattle, the effect of alternating periods of nutrient restriction during the post-weaning stage, followed by a re-feeding stage, is still unclear. In some cases, heifers that were initially restricted in their nutrient intake but were later subjected to compensatory growth reached puberty at a similar age as heifers that were always provided with 100% of their requirements56)(70, but in other cases they were delayed by a few months32)(71. Of note, in all studies the BW at which heifers reached puberty did not differ between feeding strategies. In Uruguay, it has been reported that dairy heifers subjected to a better feeding plan for 4 months after weaning achieved a greater BW gain (0.8 vs 0.6 kg/d) and tended to have higher concentrations of IGF-I during the period of application of the treatments compared to heifers under a lower feeding plane. However, heifers subjected to a higher feeding level not only did not reach puberty earlier, but on day 350 of life the proportion of animals that had reached puberty was lower compared to animals under a lower feeding plane (0.47 vs 0.78); likewise, heifers with a high feeding plane weighed 45 kg more at puberty72)(73. It should be noted that after the application of the treatments all the animals were managed with the same diet and gained 0.8 kg/d, regardless of the previous treatment; this relative improvement in weight gain for the heifers previously managed with a lower level of feeding could have been a stronger signal explaining the more rapid onset of puberty observed in those animals, similar to that reported in heifers subjected to compensatory growth, as already mentioned.
Information related to the effects of feeding level during the prenatal and pre-weaning periods on puberty development of dairy heifers is scarce and inconsistent compared to the one related to the effects of feeding level during the post-weaning period. It has been reported that heifers that were fed milk ad libitum during rearing reached puberty 23 days earlier than heifers that were restrictedly fed with milk replacer, although there were no differences in BW or various measures of skeletal development at puberty74. Others75 reported that heifers fed with a milk replacer and starter concentrate with a high concentration of energy and protein during rearing reached puberty 31 days earlier, with 20 kg less BW, and lower WH and width between hips than heifers fed a less intensive diet. In Uruguay, calves fed with 8 L/d of milk during rearing reached puberty 45 days earlier, with 28 kg less BW, but with greater width between the hips, WH, and heart girth compared with calves fed with 4 L/d; however, there were no differences in the number or diameter of follicles in the prepubertal stage. Furthermore, heifers that were offered a higher supply of milk had higher blood concentrations of IGF-I both in the lactating stage and in the post-weaning stage (when all animals received the same diet), although there was no effect of the treatments on insulin blood concentrations76.
On the other hand, increasing milk supply from 5 to 10 L/d during the pre-weaning stage and offering a post-weaning diet with a higher proportion of concentrates resulted in a higher blood concentration of leptin in heifers during both periods. While the more intensive pre-weaning diet increased the peak and duration of the LH pulses at week 15 of life and increased the number of follicles in the prepubertal stage, this did not translate into an earlier onset of puberty. Although post-weaning feeding did not affect LH secretion, heifers with a higher feeding plane at this stage had a higher number of follicles in the pre-pubertal stage and a higher probability of entering puberty at 30 weeks of age77)(78.
More recently, it has been observed that the environment in which the fetus develops can have a long-term impact on future performance, a phenomenon known as developmental programming or fetal programming50. The nutrition that the mother receives during pregnancy is a factor that can affect the uterine environment and have long-term implications for the development of the animal, including the time of attainment of puberty, an aspect observed in rats whose mothers were undernourished during pregnancy79, but which has only recently been explored in bovines. In one study80 no differences were observed in the age at puberty between heifers born to undernourished cows or not during the second and third trimesters of gestation, but others81 observed that heifers whose mothers were supplemented with protein during the last third of gestation reached puberty at a younger age than daughters of cows that were not supplemented. These differences between experiments could be related to the level of nutrient restriction imposed in each study. More recently, it has been proposed that the nutrition received by the cow during gestation interacts with the feeding plane received by the heifer after birth to program the onset of puberty51. These authors evaluated different feeding levels during the second and third trimesters of the dam's gestation and between weaning at 3 months of age and for 5 months, and reported that better postnatal nutrition, and, to a lesser extent, better prenatal nutrition were associated with a lower age at puberty in heifers. However, heifers subjected to low feeding levels in both stages took almost 100 days longer to reach puberty than those that were always managed with a high feeding plane. Although it is possible that the neuroendocrine mechanisms that explain these effects are similar to those that mediate the effects of postnatal nutrition on the onset of puberty, they are still poorly understood.
4. Social environment during the post-weaning period and pubertal development
Several factors associated with the social environment affect production, development, and welfare in ruminants82)(83)(84. Cattle are gregarious animals in which the social structure is based on hierarchy relationships85. Social dominance is the relation between two individuals, dominant (winner) and subordinate (loser), and “it refers to the condition of an individual's respect to another in the same group”86. From an evolutive point of view, social hierarchy would act as a markedly adaptive trait, allowing a better use of resources, with a minimum of destructive conflicts87. However, it can be a limitation for the performance of many animals, especially the subordinate or low ranked ones88. In this sense, if management conditions are inadequate (high animal density, reduced feeding space, uneven groups of animals), social dominance can have negative consequences on heifers' health, welfare, and productive performance9)(87)(89. Specifically, increased competence at the feeding bunk determined greater variability in ADG90)(91 and increased BW in heifers of higher rank compared to those of lower social rank92.
The information related to the effects of social dominance on reproductive performance, and especially on puberty development, is scarce. In dairy cows, both milk production and fertility increased with social rank93)(94, while high-ranked beef cows were rebred earlier during the postpartum period compared with low-ranked ones95. In male lambs, high-ranked animals had greater ADG, and a precocious increase of scrotal circumference, semen production, and sexual behavior compared to low-ranked ones96. In Uruguay, the effects of social dominance on body and reproductive development, metabolic status and behavior in dairy heifers maintained under intensive feeding systems in a competitive environment has been evaluated97)(98. These authors reported that dominant heifers presented higher BW, glucose concentrations and attained puberty earlier, compared to subordinate heifers97. Moreover, subordinate heifers had greater intake rate and behaviors linked to increased levels of social stress (less rumination and greater walking and standing behaviors) in contrast to dominant ones98. Thus, social dominance in females maintained under competitive situations represents a social stress factor that may affect both productive and reproductive performance in dairy heifers.
The establishment of social hierarchies in cattle is performed through physical encounters, and agonistic interactions99. If the social group is maintained stable, it is expected that the individual order in the social hierarchy would not change100. In contrast, when animals are subjected to social regroupings (SR), a new social order must be achieved after each SR, increasing the physical interactions and agonistic behaviors on the days after SR101)(102. In general, 3 to 14 days are necessary to reestablish the new social order after each SR99)(103)(104. Several factors, like animal category, number of individuals in the group, and competence level for resources105)(106 affect the period necessary to stabilize the social group. The changes associated with the formation of a new group and/or SR have been reported to affect behavioral pattern101, body development107 and milk production101)(108)(109)(110 in cattle. When SR are performed repeatedly, it was found that calves regrouped up to 14 times spent less time lying and more time standing only during the first SR, without differences at the following SR111. In contrast, others112 reported no habituation in heifers regrouped up to 16 times. In Uruguay, a recent study evaluated the effects of repeated SR on body development, onset of cyclic activity, metabolic status and behavior in dairy replacement heifers managed on grazing conditions in a contract-rearing farm113. It was reported that body development (BCS and WH) was temporarily affected, while the onset of cyclic activity was delayed in more than one month in heifers regrouped every 21 days for a total of 10 SR compared to those that were maintained in the same group. In addition, regrouped heifers presented lower IGF-1 and glucose concentrations, and increased NEFA concentrations, which are linked with a more negative metabolism, as well as altered ingestive and postural behavioral patterns, compared to non-regrouped ones. Thus, repeated regroupings affect productive and reproductive performance in dairy heifers, so they should be avoided or at least minimized during rearing.
5. Integrative approach
In this section, we aimed to integrate the results of the studies performed in Uruguay that evaluate the effects of different factors associated to social environment and feeding level on age at puberty in dairy heifers. For this, data of four different studies72)(76)(97)(113 were summarized (Table 1). In only one of those studies heifers were maintained in a confined system, without access to pasture97, while on the other three they grazed pastures. The final dataset contained the information of 118 animals, with the following variables: number of study, number of animals, treatment, evolution of BW, WH and HG, and concentrations of IGF-1, insulin and glucose, along the different sampling periods. Afterwards, the information contained in the dataset was classified in two groups: according to heifers that began puberty before and after 300 days of age (“early” and “late” heifers). In addition, response variables (IGF-1, insulin, glucose, BW, WH, HG) were classified according to the age of the heifers at the different sampling periods: period 1:> 0 and ≤ 120 days, period 2: > 120 and ≤ 240 days, and period 3: > 240 and ≤ 360 days of age. Data was analyzed with a mixed linear model (PROC MIXED) using SAS (SAS® On Demand for Academics; SAS Institute Inc., Cary, NC), with the age at puberty (< 300 d and> 300 d), age at sampling period (periods 1, 2 and 3) and the interaction between age at puberty and age at sampling period as main effects, and the experiment, animal and treatment as random effects. The compound symmetry covariance structure was included. In addition, simple regressions (PROC CORR) were performed between some variables of interest: age with BW at puberty, and ADG with age at puberty and BW at puberty. Average daily gains were calculated as the differences between BW at birth and BW at puberty. Significant differences were considered when P≤ 0.05 and tendencies as 0.05 <P≤ 0.1. The results are presented as mean ± SEM.
Table 1: Descriptive information of the studies that evaluated different factors that affect the onset of puberty in dairy heifers in Uruguay
| Study | Number of animals per treatment | Treatment period1 | Treatment type2 | Total duration, d3 | Age at the beginning, d4 |
| De Trinidad76 | 34 | PreW (56 d) | High vs Low | 416 | 4 |
| De La Quintana72 | 40 | PostW (120 d) | High vs Low | 270 | 78 |
| Fiol and others97 | 16 | PostW | Dom vs Sub | 120 | 250 |
| Moratorio and others (113 | 28 | PostW | RG vs non-RG | 205 | 270 |
1 PreW: the treatments were applied during the pre-weaning period. PostW: the treatments were applied during the post weaning period. 2 Periods when the treatments were applied. High vs Low: two feeding levels were evaluated. Dom vs Sub: heifers maintained in dyads were classified according to social dominance. RG vs non-RG: heifers were regrouped with 5 new heifers every 21 days or maintained in a stable group for 210 days. 3 Total duration of the experimental periods, including the periods when the treatments were applied. 4Age of the females at the beginning of the studies.
Age and BW at puberty presented a high positive linear correlation (r = 0.56, P < 0.01; Figure 1), which is in agreement with studies that reported that heifers maintained under different managements reached puberty with different BW16)(32, and in contrast to the hypothesis that cyclic activity begins at the moment that heifers reach a target BW28)(29)(30. The correlation between ADG and age at puberty was also very high and negative (r = -0.58, P< 0.01; Figure 2a), while the correlation between ADG and BW at puberty was lower and positive (r = 0.33, P< 0.01; Figure 2b). In this sense, as previously reported in dairy cattle56)(57)(58, as heifers gained more BW, the age at puberty decreased, which implies a relevant effect of nutritional level and/or factors that determined changes on feeding intake (e.g. social environment) during females rearing on the onset of cyclic activity.
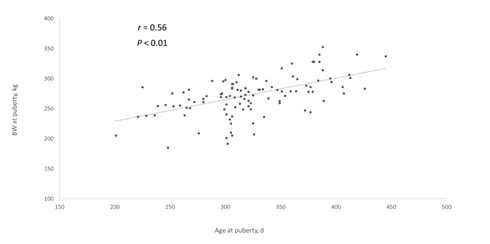
Figure 1: Correlation between age and body weight at puberty in dairy heifers from a dataset of four studies performed in Uruguay (n =118 heifers)
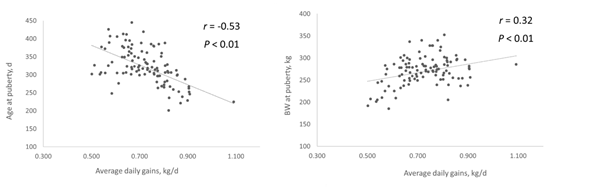
Figure 2: Correlations between average daily gains from birth to puberty with age (a) and body weight (BW); b) at puberty in dairy heifers from a dataset of four studies performed in Uruguay (n =118 heifers)
Heart girth tended to be greater in “early” compared to “late” heifers (P = 0.10; Table 2), while no effect of age at puberty was found for any of the other variables evaluated, but an interaction between age at puberty and age at sampling was found for BW, WH, HG, and glucose, and a tendency for IGF-1 (Table 2). On period 1, BW was greater in those heifers that attained puberty younger (54.4 and 71.0 ± 12.4 kg, “early” and “late” heifers, respectively; P< 0.01), while on period 3 “early” heifers presented a greater BW compared to the ones that began puberty later (264.8 and 245.0 ± 12.3 kg, “early” and “late” heifers, respectively; P< 0.01). Thus, heifers that began puberty earlier were lighter during the preweaning and immediately after weaning periods, but they had greater postweaning ADG, which determined a greater BW at the time around puberty, compared to those that were older at puberty. Interestingly, heifers that attained puberty younger tended to present greater IGF-1 concentrations on period 1 (171.3 and 143.4 ± 49.0 ng/ml, “early” and “late” heifers, respectively; P = 0.06) compared to those that were older at puberty. In addition, glucose concentrations tended to be greater (P = 0.1) in “early” compared to “late” heifers on period 1 (5.1 and 4.9 ± 0.2 mmol/l, “early” and “late” heifers, respectively). In this sense, although heifers that were older at puberty presented greater BW at period 1, IGF-1 and glucose concentrations were greater in heifers that were younger at puberty during the same period, which may be associated to differences in some metabolic signals between both types of females. In fact, previous studies reported that elevated concentrations of metabolic signals, such as IGF-1, during early stages of life promote changes within the neuroendocrine system that may affect puberty onset51. On periods 2 and 3, both WH (period 2: 101.8 and 100.2 ± 2.0 cm; period 3: 115.7 and 114.3 ± 2.0 cm, for “early” and “late” heifers, respectively; P< 0.01) and HG (period 2: 128.0 and 124.5 ± 1.3 cm; period 3: 150.4 and 147.5 ± 1.4 cm, for “early” and “late” heifers, respectively; P< 0.01) were greater in heifers that attained puberty early compared to those that were older at the beginning of cyclic activity. Thus, greater skeletal development may be linked to reduced risk of calving problems and adequate mammary gland development on precocious heifers52)(53.
Table 2: Body development, IGF-1, insulin, and glucose concentrations (mean ± EEM) in dairy heifers that attained puberty before and after 300 days of age, and according to age at sampling in a dataset of four different studies performed in Uruguay
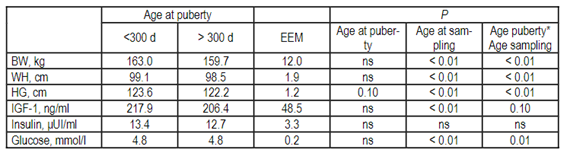
BW: Body weight. WH: Withers height. HG: Heart girth. Age at sampling: period 1: > 0 and ≤ 120 days, period 2:> 120 and ≤ 240 days and period 3: > 240 and ≤ 360 days of age.
6. Final conclusions and future trends
While the effect of post-weaning feeding level on the onset of puberty has been well studied, the impact of alternating periods of restriction and subsequent acceleration of growth (i.e. compensatory growth) on dairy heifer rearing, or the level of ADG that allows an early onset of puberty without compromising mammary gland development still need to be defined. Likewise, more information is needed to evaluate the effects of the pre-weaning or pre-natal feeding level on the onset of puberty, where although promising results have been reported, the information is still scarce and inconsistent. Moreover, it is also necessary to know how they interact with the feeding level that the animals receive after weaning. The promising results about the effects of the manipulation of the social environment on the attainment of puberty in heifers should encourage to continue exploring this research area, particularly in what has to do with the effects of competition and regrouping of animals on early stages of life, and how these aspects can modulate the response to feeding management. In addition, studies should consider and evaluate the possible effects of the social environment on animal welfare.
The particularities of the heifer rearing system in Uruguay, in the open field and with a diet mainly based on pastures, impose some restrictions (e.g. under-nutrition during times of forage scarcity, heat stress), which lead to the need to study which components of the production systems could be modified to achieve the goals of growth and body development, which in the present are not achieved by the average of the farms. It would be of interest to evaluate the possibility of modifying the forage base and grazing systems, the inclusion of specific feeds or additives that may enhance growth, the provision of shade, or the partial confinement of the animals in those critical periods, so as not to compromise the onset of puberty.
Finally, studies at the level of the production system would be desirable to verify that an earlier attainment of puberty in heifers has a positive impact on fertility at first service and age at first calving, without affecting the productive life of the animal. These studies should consider not only biophysical aspects but also economic and environmental ones. Regarding this last point, the reduction in the age at first calving in heifers with an adequate development, which depends on an early attainment of puberty, reduces the number of replacement animals that need to be kept on the farm, which would result in a lower emission of greenhouse gasses114. Considering that the stock of replacement animals contributes with 21-26% of the enteric CH4 produced by the entire herd115, the contribution of lowering the age at first calving could be an interesting tool to mitigate the effects of milk production on the environment.















