1. Introduction
The potential of Teramnus labialis (L.f.) Spreng to be used as animal feed1 and cover crop2 has been demonstrated by several researchers. However, currently it has not been possible to extend its use mainly due to the presence of physical dormancy in the seeds 3)(4) 5 and the slow growth of seedlings in the initial stage of field establishment6.
The slow growth of seedlings is related to the small size of the seeds and the low availability of reserves necessary for emergence and initial growth4, which is accentuated during seed storage 7)(8) 9. During the storage period, seeds of many species experience loss of viability and vigor 10)(11)12)(13) ; this phenomenon being observed in T. labialis seeds8.
Viable seeds, with high vigor, provide synchronized germination and the ability to emerge and survive under adverse environmental conditions 14)(15) . On the contrary, in seeds that experience certain deterioration or loss of vigor, some of the advantages explained above are suppressed. However, there are several pre-germinative treatments to reinvigorate seeds that are defined as seed priming 16)(17) and these are: water conditioning18, solid matrix conditioning19, osmotic conditioning20, chemical conditioning21, hormonal conditioning22, thermal conditioning23, nanoparticle conditioning24 and organic conditioning 25)(26) .
For organic priming, several conditioning agents are used, including: garlic extracts, algae, green tea, protein hydrolysates, food industry waste and different microorganisms 25)(26) 27. In this sense, various efficient microorganisms have been used for the conditioning of seeds of Dacus carota L. and Allium sativum L. 28)(29) , Brassica oleracea L.30 and Triticum spp. and Zea mays L.31. In Cuba, the formulation of efficient microorganisms IHPLUSTM has been used for seed priming in crops such as: Sorghum bicolor L. (Moench), Zea mays L. and Cucumis sativus 32)(33) , showing great effectiveness in the germination and reinvigoration of seeds. However, there is no reference in the reviewed literature of studies related to seed priming in the T. labialis species to enhance germination and initial growth of seedlings. Therefore, the present research aims to determine the effectiveness of IHPLUSTM for the priming of T. labialis seeds.
2. Materials and methods
Mature seeds were collected from 50 plants of Teramnus labialis (L.f.) Spreng grown in Ciego de Ávila, Cuba (21˚50`25.10"N, 78˚45`32.24"W). The seeds had humidity content of 7.96%, a viability of 98.00%, 95.00% of high vigor and 3.00% of low vigor at the time of harvest. The seeds were stored in artisanal conditions according to Acosta and others8, during twelve months in hermetically sealed glass containers.
2.1 Seeds quality after stored
To estimate viability and vigor, the topographic test of 1% tetrazolium (2, 3, 5 triphenyl-2 H-tetrazolium chloride) was used as described by ISTA34. Seeds were classified as per the degree of coloration as: (1) viable with high vigor, when they were completely stained deep red; (2) viable with low vigor, when the coloration was light red or with colorless sections, and (3) non-viable without vigor, when they remained the natural coloration35.
2.2 Seeds priming
Before seeds priming, mechanical scarification was carried out in seeds to homogenize and promote imbibition and germination according to Acosta and others3. Two thousand seeds were used, one thousand for control treatments (seeds without priming) and one thousand for priming with efficient microorganism. For the seeds priming, a formulation of the efficient microorganism IHPLUS TM(32) was used. The seeds of this treatment were immersed directly in a Biker with 50 mL of IHPLUSTM at a concentration of 5% (v:v) and for 3 h according to Bolaño and others36. After 3 h, the seeds were extracted and rinsed with distilled water for 1 minute to eliminate any remains of IHPLUSTM impregnated on the outside of the seed coat and then dried for 30 minutes on felt paper at a temperature of 25 ± 1 °C. Subsequently, the seeds without priming (control) and those with IHPLUSTM priming were used in the following research experiments.
2.3 Evaluations during germination
Germination (%): For each treatment (seeds without priming (control) and seeds priming with IHPLUSTM) four repetitions of 25 seeds were used (n = 4). The seeds were placed to germinate in Petri dishes (9 cm in diameter) on filter paper (FILTRAK) previously moistened with 5 mL of distilled H2O and placed in a controlled environment pre-germination chamber (RTOP-D Series) at a temperature of 30±1 °C for 7 days. After this period of time, the germination percentage (%) was determined and some numerical variables associated with vigor were calculated: time necessary for 50% of the seeds to germinate (T50), germination index (GI), mean germination time (MGT) and germination synchrony (Z) according to Ranal and others37.
Amino acid content: Three repetitions of 60 seeds (n = 3) were used for each treatment (seeds without priming (control) and seeds priming with IHPLUSTM). The seeds were placed to germinate in Petri dishes (9 cm in diameter) on filter paper (FILTRAK) previously moistened with 5 mL of distilled H2O and placed in a controlled environment pre-germination chamber (RTOP-D Series) at a temperature of 30±1 °C for 24 h. At the beginning (0 h) and at 12 and 24 h, seed samples were taken for each treatment (20 seeds per repetition) to determine the amino acid content according to Moore and Stein38.
2.4 Evaluations on seedlings after 14 days of cultivation
For the cultivation of seedlings, two samples of 300 seeds were taken for each treatment (seeds without priming (control) and seeds priming with IHPLUSTM) to place them to germinate in polyurethane trays with 136 alveoli. The trays were filled with a substrate based on Typical Red Ferralitic soil (50%) and worm humus (50%) and one seed was sown for each alveolus at a depth of 2 cm, based on that recommended by Machado and Roche39. Next, the trays were placed in the growing house in semi-controlled conditions and daily watering was maintained for 14 days.
Biochemical determinations: To carry out the biochemical determinations of the seedlings 14 days after sowing, the aerial part (leaves and stems) of 30 seedlings (n = 30) for each treatment (10 per repetition) was taken. The content of chlorophyll a, b and total (µg g fresh mass-1) was determined in both the leaves and stems according to the methodology described by Porra 40) and the nitrogen content (%) and proteins (%) by the Kjeldahl method41.
Physiological determinations: To carry out the physiological determinations of the seedlings 14 days after sowing, 30 seedlings were taken for each treatment (10 per repetition) (n = 3) and the fresh and the dry mass (g) of the seedling were determined.
Morphological determinations: To carry out morphological determinations of the seedlings 14 days after sowing, 30 seedlings (n = 30) were taken for each treatment (10 per repetition) and the number of leaves per seedling, stem length (cm), stem thickness (cm), root length (cm) and root thickness (cm) were determined.
3. Results
3.1 Seeds quality after stored
The moisture content of T. labialis seeds after storing them for 12 months was 7.74% and decreased slightly in relation to the time of harvest (7.96%). However, viability decreased to 93.00% (98.00% at the time of harvest) and the percentage of seeds with high vigor to 73.00% (95.00% at the time of harvest), which shows some degree of deterioration during storage (Table 1).
3.2 Evaluations during germination
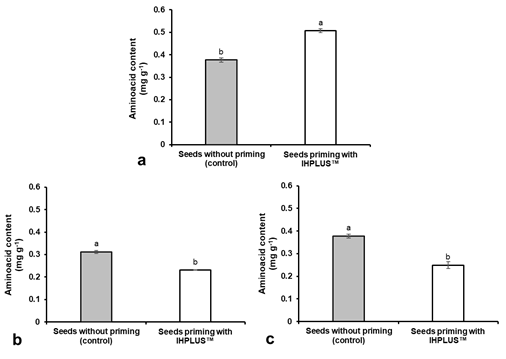
Seeds priming with IHPLUSTM significantly improved the germination percentage in controlled conditions and all the variables associated with vigor (Figure 1, Table 2). Additionally, an increase in the amino acid content was observed in the seeds conditioned at 0 h (Figure 2). However, it was observed that the amino acid content decreased significantly in the conditioned seeds during germination at 12 h and 24 h compared to those of the control treatment.
Table 2: Effect of IHPLUSTM on germination and some mathematical variables associated with the vigor of T. labialis seeds during germination in controlled conditions
| Seeds without priming (control) | Seeds priming with IHPLUS™ | |
| Germination (%) | 72.00 ± 2.2 (b) | 89.00 ± 2.3 (a) |
| Germination index (seeds day-1) | 7.06 ± 0.48 (b) | 10.75 ± 0.54 (a) |
| Time required for germination of 50% of the seeds (days) | 3.5 ± 0.12 (a) | 0.83 ± 0.04 (b) |
| Mean germination time (days) | 3.45 ± 0.22 (a) | 2.44 ± 0.16 (b) |
| Germination synchrony | 0.23 ± 0.01 (b) | 0.56 ± 0.03 (a) |
Results with unequal letters, in each row, are statistically different (t-test, P ≤ 0.05). Ranges indicate the mean ± standard error of the mean.
3.3 Evaluations on seedlings after 14 days of cultivation
The content of chlorophyll a, b and total 14 days after sowing the seeds did not show significant differences between the treatments evaluated (Table 3). However, the content of total nitrogen and total protein, in the same period of time, increased significantly in the aerial part (leaves and stems) of the seedlings obtained from seeds priming with IHPLUSTM. Likewise, fresh and dry mass, the number of leaves per plant, the length and thickness of the stem, and the length and thickness of the root increased significantly in the seedlings obtained from seeds priming with IHPLUSTM after 14 days of cultivation (Table 3).
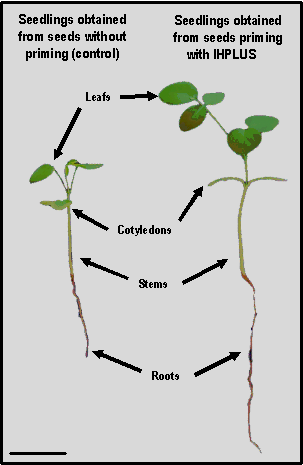
The increase in fresh and dry mass indicators is closely related to the growth of seedlings 14 days after sowing. As can be seen in Figure 3, when the seeds are primed with IHPLUSTM, more vigorous and larger plants are obtained. This result is closely related and is a consequence of the increase in some morphological characters such as stem length and the number of leaves per seedling.
Table 3: Determinations on T. labialis seedlings after 14 days of growth in semi-controlled conditions
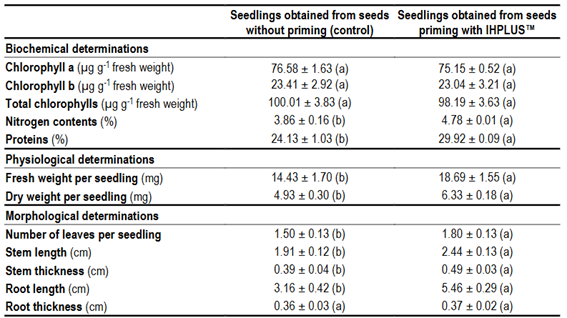
Results with unequal letters, in each row, are statistically different (t-test, P ≤ 0.05). Ranges indicate the mean ± standard error of the mean.
4. Discussion
T. labialis seeds showed deterioration during storage under artisanal conditions, which coincides with what was described for this species by Acosta and others8. Varghese and Naithani 42) reported that deterioration during storage is due to a gradual increase in reactive substances of thiobarbituric acid, resulting from the progressive accumulation of reactive oxygen species. An aspect also described by Walters10, who suggests that this is due to the temperature and humidity conditions prevailing during storage, which lead to a loss of viability and vigor in the seeds.
The priming of T. labialis seeds caused an increase in the germination percentage and the reinvigoration of the seeds. This is because seed priming causes an increase in the activity of enzymes involved in the respiration and catabolism of starch, proteins and lipids, resulting in more rapid mobilization of reserves to the growing points 43)(44) . Specifically, the results of this research suggest the entry into the interior of the seeds of substances that stimulate enzymatic activity. This influences greater efficiency in metabolic processes, increasing the speed of germination and reinvigoration of the seeds 45)(46) . As a result, an increase in the amino acid content was observed at 0 h in the primed seeds, but with a decrease at 12 and 24 h after they were put to germinate. Torres and others47 indicated that the results obtained during the germination of Cajanus Cajan seeds showed a reduction in amino acids at a time close to the emergence of the radicle since they were used in the synthesis of new proteins. These proteins are formed with the aim of providing energy through the oxidation of their structures to promote the growth and elongation of the embryo48.
It is known that the amino acid content varies during germination in legume seeds49. Some amino acids also act as precursors for the biosynthesis of spermidine and gibberellin, growth regulators, and many secondary metabolites50. The mobilization and transformation of reserves accumulated in seeds are crucial for the efficiency of germination and seedling establishment51. In conditioned T. labialis seeds, during germination, the amino acid levels do not present differences in the first 24 hours. This implies that germination occurred in a shorter period and in a higher percentage in seeds priming with IHPLUSTM. Presumably, these levels of amino acids were provided to the seeds by the bioproduct used for priming. Amino acids act as a regulator of cytokinins and auxins, improving the elongation and growth of roots, which contributes to the absorption of nutrients by the new seedling52. Previously, it was observed that seeds primed increase shoot and root length, and fresh and dry mass of seedlings in rice crops53 and wheat crops 54)(55) . Furthermore, it was observed that the germination rate, the final germination and the seedling size improved in wheat cultivation56. Likewise, increases in the vigor index were also demonstrated in response to seed primed in rice and carrot crops 57)(58) .
Different studies related to primed seeds showed greater efficiency in nitrogen use, which can be reflected in resistance to phytopathogens and in vegetative development of seedlings 59)(60) 61. The length and diameter of the stem are indicators of the vigor of the seedling, which is considered important since they show the strength and resistance it may have when subjected to field conditions62. The differences observed between seedlings obtained from seeds primed with IHPLUSTM and the control may be associated with the hormonal balance between the different endogenous and exogenous growth regulators and the nitrogen contents shown. Díaz and others63 attribute this effect to biostimulant action mechanisms, such as auxinic amino acids, which coincides with Castillo-Portela and others64, who also refer to this effect of the hormones that intervene in the growth of the different parts of the seedlings. In this sense, Batista-Sánchez and others65 reported that biostimulants contain amino acid and carbohydrate chelates that are rapidly absorbed, have greater mobility once absorbed, and possess root growth-stimulating properties.
Various researchers observed an improvement in the number of leaves in corn crops66, in leaf area in banana crops67, in fresh and dry mass of leaves in wheat68, and in fresh and dry mass in chickpea and wheat seedlings69, in response to seed conditioning. The use of IHPLUSTM for the priming of Sorghum bicolor seeds stimulated the growth of the roots and the aerial part of the seedlings33. Meena and others59, when investigating Trichoderma harzianum as a conditioning agent for wheat seeds, reported increases in seedling height, root length, field yield and chlorophyll content.
5. Conclusions
The use of IHPLUSTM for priming of T. labialis seeds stored for 12 months increased the germination percentage under controlled laboratory conditions. A reinvigorating effect was observed in the conditioned seeds, which was expressed in a better mobilization of amino acids during germination. In seedlings grown up to 14 days under semi-controlled conditions, nitrogen and protein content, fresh and dry mass, and vegetative development were significantly higher when the seeds were conditioned with IHPLUSTM.