Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.26 no.43 Montevideo 2024 Epub June 01, 2024
https://doi.org/10.22592/ode2024n43e233
Research
Bacterial biofilm formation on the surface of PEEK and titanium healing abutments
1Programa de Magister en Ciencias Odontológicas, Facultad de Odontología, Universidad de Concepción, Chile.
2Departamento de Odontología Restauradora, Facultad de Odontología, Universidad de Concepción, Chile.
3Departamento de Prevención y Salud Pública, Facultad de Odontología, Universidad de Concepción, Chile. Autor de correspondencia: Dr. Michael Wendler. Roosevelt 1550, 4070369, Chile mwendler@udec.cl
4Facultad de Ciencias para el Cuidado de la Salud, Universidad San Sebastián, Campus Las Tres Pascualas, Concepción, Chile.
Objectives
. To explore the effect of surface characteristics on the total volume and viability of a bacterial biofilm developed on the surface of PEEK and titanium healing abutments.
Methods.
Surface parameters S a and S k, as well as the surface energy of PEEK and titanium healing abutments (n=3) were determined using confocal laser scanning microscopy (CLSM) and contact angle, respectively. The total volume and viability of a multispecies bacterial biofilm cultivated for 30 days were determined using CLSM and the LIVE/DEAD BacLight reactive kit. Effect size was determined using Cohen’s d.
Results.
PEEK healing abutments displayed a higher surface roughness than titanium (S a 0.41 µm vs 0.17 µm), although no differences in surface energy were observed. Despite the higher total volume of the biofilm measured on titanium abutments compared to PEEK (696 µm3 vs 419 µm3), no differences in the live/dead bacterial ratio were observed.
Conclusions.
Bacterial viability of the biofilm did not show a direct relation to the surface characteristics of PEEK and titanium healing abutments.
Keywords (MeSH, DeCS): dental abutment; titanium; PEEK; biofilms; microbial viability
Objetivos.
Explorar el efecto de las características de superficie sobre el volumen total y la viabilidad de la biopelícula formada sobre pilares de cicatrización de PEEK y titanio.
Métodos.
Los parámetros de rugosidad (S a y S k) y la energía superficial de pilares de cicatrización de PEEK y titanio (n=3) fueron determinados mediante microscopía confocal láser de barrido (CLSM) y ángulo de contacto, respectivamente. Se determinó luego el volumen total y la viabilidad de una biopelícula bacteriana multiespecie cultivada por 30 días, mediante CLSM y el reactivo LIVE/DEAD Kit BacLight. El tamaño del efecto se determinó mediante d de Cohen.
Resultados.
Los pilares de PEEK mostraron una mayor rugosidad que los de titanio (S a 0,41 µm vs 0,17 µm), pero no se observaron diferencias en la energía superficial. Si bien el volumen total de biopelícula fue mayor en titanio que en PEEK (696 µm3 vs 419 µm3), no hubo diferencias en la proporción de bacterias vivas entre ambos materiales.
Conclusiones.
La viabilidad de la biopelícula bacteriana formada no guarda relación directa con las características superficiales de pilares de cicatrización de PEEK y titanio.
Palabras clave: (MeSH; DeCS); pilar dental; titanio; PEEK; biopelículas; viabilidad microbiana
Objetivo.
Explorar o efeito das características da superfície no volume total e viabilidade do biofilme formado em PEEK e pilares de cicatrização de titânio.
Métodos.
Parâmetros de rugosidade (S a e S k) e energia de superfície de PEEK e pilares de titânio (n = 3) foram determinados por microscopia confocal de varredura a laser (CLSM) e ângulo de contato, respectivamente. O volume total e a viabilidade de um biofilme bacteriano multiespécie cultivado por 30 dias foram então determinados usando CLSM e o reagente LIVE/DEAD Kit BacLight. O tamanho do efeito foi determinado usando o d de Cohen.
Resultados.
Os pilares de PEEK mostraram maior rugosidade do que os de titânio (S a 0,41 µm vs 0,17 µm), mas não foram observadas diferenças na energia de superfície. Embora o volume total de biofilme tenha sido maior no titânio do que no PEEK (696 µm3 vs 419 µm3), não houve diferenças na proporção de bactérias vivas entre os dois materiais.
Conclusões.
A viabilidade do biofilme bacteriano formado não está diretamente relacionada às características da superfície dos pilares de cicatrização de PEEK e titânio.
Palavras-chave: (MeSH; DeCS); pilar dentário; titânio; PEEK; biofilmes; viabilidade microbiana
Introduction and background:
Dental implants are crucial components in the restorative treatment of partially and completely edentulous patients. Following surgical placement, the supportive and protective tissues initiate a healing process that ultimately leads to the biological and functional integration of the implant into the host. In the case of peri-implant soft tissues, this process is guided by the use of healing abutments and temporary prostheses, which also play a significant role in establishing the microbiota in the new peri-implant environment.
Healing abutments can be fabricated from various materials, with titanium, polymethyl-methyl-methacrylate (PMMA), and polyetheretheretherketone (PEEK) being the most common. PEEK, in particular, has garnered attention in recent years due to its physicochemical and mechanical properties, which have been observed to promote healing and tissue evolution around the implant 1-3. Moreover, it exhibits superior aesthetic performance compared to metal alloys, thereby encouraging its use in aesthetically challenging areas (4. Titanium, on the other hand, exhibits excellent mechanical and physicochemical properties, as well as well-known biological compatibility, attributed to its high chemical stability and corrosion resistance, among other factors 5. However, some authors have noted its inferior antibacterial and anti-adherent capabilities compared to zirconia 6 or PEEK (7.
Features influencing biofilm formation on abutments include the roughness of its surface, its surface energy, as well as intrinsic features of the materials used to manufacture it (8-10. However, the specific role of each feature, particularly the material's effect on biofilm formation and viability, remains unclear. Therefore, the objective of this descriptive study is to explore the effect of surface and material characteristics on the total volume and viability of bacterial biofilm developed on PEEK and titanium healing abutments.
Specimen Preparation
Four titanium healing abutments (Ø: 6.5mm; H: 6mm; RC; RC platform Ø: 4.1mm) and four PEEK healing abutments (Ø: 5mm; H: 7mm; NC; NC platform Ø: 3.3mm) from the Straumann/Neodent company (Straumann®, Basel, Switzerland) were prepared. The upper cylindrical portion of each abutment was separated from its base using a high-precision saw (IsoMet 1000, Buehler, Illinois, USA) with continuous irrigation, as depicted in Figure 1.

Figure 1. On the left, the titanium abutment, on the right, the PEEK abutment. The upper portion was used, which was sectioned from its base (dotted line)
Surface Roughness
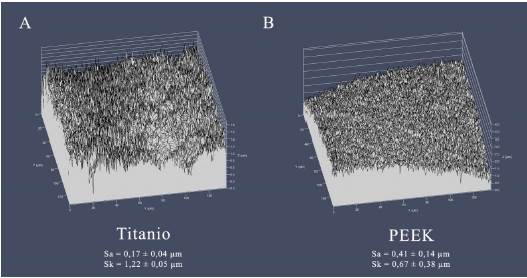
Surface roughness was assessed using Confocal Laser Scanning Microscopy (CLSM) in reflection mode (LSM 780, Zeiss, Jena, Germany) on random areas at the top of each abutment. Areal roughness parameters were analyzed for each surface (arithmetic average roughness, Sa, and skewness, Sk).
Surface Energy
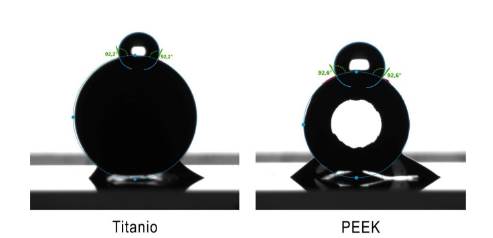
Surface energy was determined by measuring the contact angle of each material using the sessile drop technique, with distilled water as the liquid. A 0.5 μL drop was poured onto the outer convex area of the abutments (Figure 2). Hydrophobicity was evaluated by measuring the angle formed by the drop with the abutment surface.
Microbiological Analysis: Total Biofilm Volume and Bacterial Viability
Three samples in each group were sterilized under ultraviolet (UV) light for 15 minutes per side and were maintained in an incubator at 37°C, mimicking oral cavity conditions.
For the preparation of the multispecies biofilm, strains (American Type Culture Collection, ATCC, Manassas, VA, USA) of Streptococcus sanguinis (ATCC 10556TM), Prevotella melaninogenica (ATCC 25845TM), Porphyromonas gingivalis (ATCC 33277TM), Streptococcus mutans (ATCC 25175TM), and Enterococcus faecalis (ATCC 29212TM) were employed. The standard inoculum for each bacterial strain was adjusted to a turbidity equivalent to 0.5 on the McFarland scale, as measured by an Oxoid turbidity meter (Fisher Scientific Company, Ottawa, Canada) 11.
The cultures of the 5 bacterial species were evenly mixed. Then, 150 μL of the mixed suspension was extracted and placed in each of the dishes containing the samples. Additionally, 1 μg /mL of hemin, 1 μg /mL of vitamin K, 3 μg /mL of yeast, and 10 μg /mL of sucrose were added to the medium. Subsequently, the samples were incubated for 30 days, all at 37 °C under anaerobic conditions. The medium was refreshed twice a week. On the final day, all samples were transferred to fluorescence microscopy dishes (FluoroDish Cell Culture Dish, 35 mm, World Precision Instruments WPI, UK) to minimize the biofilm's exposure to oxygen as much as possible.
To determine bacterial viability, the LIVE/DEAD Kit BacLight reagent (Invitrogen, Carlsbad, USA) was used. Initially, a 2X stock solution of the reagent mixture was prepared following the manufacturer's instructions. The contents of pipette component A (SYTO 9 stain) and pipette component B (Propidium iodide) were dissolved in a common volume of 5 mL of filter-sterilized water. The solution was thoroughly shaken until a homogeneous mixture was achieved. Subsequently, samples were stained with 500 μL of LIVE/DEAD Kit BacLight reagent and covered with aluminum foil for 10 minutes.
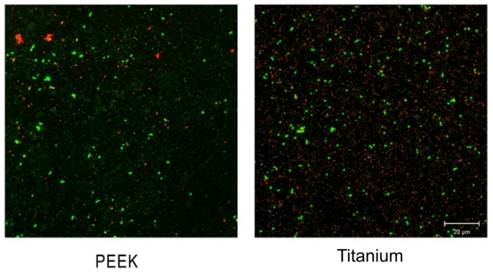
The samples were subjected to CLSM analysis using an argon laser, with excitation at 488 nm and an acquisition spectrum adjusted to the following parameters: 490-560 nm for green and 560-639 nm for red (ZEN Software 2011). Images of each sample were obtained using a 63X immersion objective. Cells with compromised cell walls allowed the passage of Propidium Iodide, staining them red due to their higher affinity for DNA than SYTO 9, enabling their identification as dead bacteria. Conversely, living cells, with intact cell walls, stained green, thanks to SYTO 9's ability to penetrate intact membranes.
Statistical Analysis
Statistical analysis was conducted using Minitab 17 (Minitab Inc., USA). Due to the limited sample size, only descriptive statistics were performed. Effect size was estimated using Cohen's d, based on the differences in group means and weighted standard deviation, to determine if there is an effect, and whether it is small, moderate, or large (12.
Results
Surface Characteristics
Roughness and contact angle values are detailed in Table 1. Figure 3 illustrates characteristic images obtained with the confocal microscope of each surface.
Table 1. Average values, with their respective standard deviations in parentheses, for roughness and contact angle
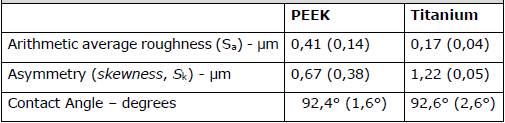
According to Cohen's criterion, the type of material had a significant effect (d < 0.2) of the material type on the contact angle was noted (Figure 2).
Total biofilm volume and bacterial viability
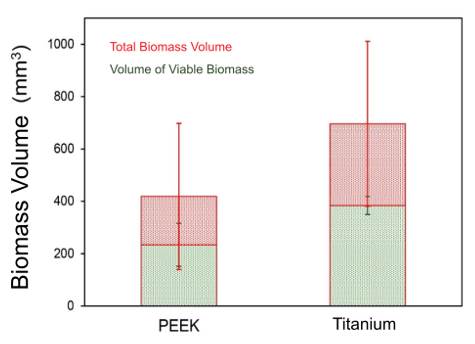
Confocal microscopy images revealed a higher volume of bacterial biofilm on titanium surfaces (696 µm3 ± 315 µm3) compared to PEEK (419 µm3 ± 279 µm3), as depicted in Figure 4. These values allow estimating a medium to large effect according to Cohen (d = -0.67). Additionally, titanium healing abutments exhibited a greater volume of live bacteria (384 µm3 ± 97 µm3) than the PEEK ones (234 µm3 ± 82.2 µm3). Nevertheless, the ratio of live bacteria to the total observed bacteria was similar (55% vs. 56% for titanium and PEEK, respectively), as illustrated in the CLSM images presented in Figure 5.
Discussion
This descriptive study took an exploratory approach to investigate the impact of material and surface characteristics on the development and viability of bacterial biofilm. While the extrapolation of the findings is constrained by the small number of evaluated samples, the observed trend of lower total bacteria volume on PEEK healing abutments’ surfaces is noteworthy for their indication and use as an alternative to titanium ones.
The development and growth of bacterial biofilm on a surface are influenced by several factors, including surface topography, hydrophobicity, surface energy, and charge 13. Studies have shown that both, an increase in roughness (Sa) exceeding 0.2 µm 14, and a higher surface energy promote biofilm formation in restorative materials, although the effect of roughness is more decisive 15. This relationship has been proven across different materials, such as composite resin 16, vitreous ceramics 17, and titanium 18. The presence of deeper and wider depressions increases the contact area, enhancing bacteria protection against external removal forces. This creates a more favorable surface for biofilm colonization and growth 19. In this study, despite observing larger roughness on the PEEK abutments compared to the titanium ones (Table 1), it was the latter that exhibited a larger total volume of biofilm on their surfaces (Figure 4). However, both materials showed biofilms with similar bacterial viability (Figure 5). In vitro studies examining these same materials have yielded disparate results. While Barkamo et al. also measured higher roughness (Sa) in PEEK than in titanium, they observed larger biofilm formation on PEEK, especially for strains like S. sanguis and S. oralis (9. Interestingly, in the same study, measurements after 120 hours of culture showed no significant differences in the biofilms of the different materials 9.
Similarly, Hahnel et al. found no significant differences in the viable biomass of PEEK, titanium, or zirconia surfaces, despite PEEK exhibiting the lowest roughness values among the materials studied (20.
On the other hand, D'Ercole et al. noted significantly higher nanoroughness on PEEK compared to both machined and etched titanium. However, the total volume of bacteria and viable bacteria (S. oralis) was notably lower on PEEK surfaces (7. Lastly, despite the absence of differences in the surface roughness of titanium and PEEK, Peng et al. identified a substantially higher total biomass (S. mutans, A. actinomycetemcomitans) on titanium than on PEEK- although, similar to the outcomes of this study, both materials exhibited similar cell viability in their biofilms 21.
Regarding surface energy, the abutments of both materials examined here were predominantly hydrophobic (contact angles exceeding 90°), albeit with very similar values between them (Figure 2). Literature reports on this property vary considerably, with some authors noting higher hydrophobicity for titanium 7, while others for PEEK (9, 20, 21). However, there is no consensus among them regarding their impact on the quantity and viability of formed biomass. It appears that the ability to promote or inhibit biofilm formation is not solely or predominantly linked to a surface characteristic but rather to a combination thereof, also highly contingent on the bacterial composition of the colonizing biofilm 19.
While the results of this study are not directly applicable to clinical scenarios, they do provide insight into how both the nature of the material and surface characteristics influence the formation and development of bacterial biofilms. When considering healing abutments, designed for relatively short-term use, their impact on the longevity of final restorations may not appear significant at first glance. However, the establishment of a potentially pathogenic microbiota in the peri-implant niche, facilitated by the characteristics of the healing abutment utilized, could influence the subsequent development of peri-implant diseases and treatment success. Hence, studies of greater complexity and scope are needed to elucidate the role of this early microbiota in such diseases.
REFERENCES
1. Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845-69. [ Links ]
2. Ramenzoni LL, Attin T, Schmidlin PR. In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation. Materials (Basel, Switzerland). 2019;12(9). [ Links ]
3. Najeeb S, Zafar MS, Khurshid Z, Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J Prosthodont Res. 2016;60(1):12-9. [ Links ]
4. Rahmitasari F, Ishida Y, Kurahashi K, Matsuda T, Watanabe M, Ichikawa T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent J (Basel). 2017;5(4) [ Links ]
5. Wheelis SE, Wilson TG, Jr., Valderrama P, Rodrigues DC. Surface characterization of titanium implant healing abutments before and after placement. Clin Implant Dent Relat Res. 2018;20(2):180-90. [ Links ]
6. Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, et al. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J Periodontol. 2017;88(3):298-307. [ Links ]
7. D'Ercole S, Cellini L, Pilato S, Di Lodovico S, Iezzi G, Piattelli A, et al. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J Mater Sci Mater Med. 2020;31(10):84. [ Links ]
8. de Avila ED, Avila-Campos MJ, Vergani CE, Spolidório DM, Mollo Fde A, Jr. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J Prosthet Dent. 2016;115(4):428-36. [ Links ]
9. Barkarmo S, Longhorn D, Leer K, Johansson CB, Stenport V, Franco-Tabares S, et al. Biofilm formation on polyetheretherketone and titanium surfaces. Clin Exp Dent. 2019;5(4):427-37. [ Links ]
10. Hao Y, Huang X, Zhou X, Li M, Ren B, Peng X, et al. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int J Mol Sci. 2018;19(10):3157. [ Links ]
11. Jerez-Olate C, Araya N, Alcántara R, Luengo L, Bello-Toledo H, González-Rocha G, et al. In vitro antibacterial activity of endodontic bioceramic materials against dual and multispecies aerobic-anaerobic biofilm models. Aust Endod J. 2022;48(3):465-72. [ Links ]
12. Cohen J. Statistical power analysis for the behavioral sciences. Second edition.1998. [ Links ]
13. Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89(7):657-65. [ Links ]
14. Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13(4):258-69. [ Links ]
15. Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17 Suppl 2:68-81. [ Links ]
16. Ionescu A, Wutscher E, Brambilla E, Schneider-Feyrer S, Giessibl FJ, Hahnel S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur J Oral Sci. 2012;120(5):458-65. [ Links ]
17. Haralur SB. Evaluation of efficiency of manual polishing over autoglazed and overglazed porcelain and its effect on plaque accumulation. J Adv Prosthodont. 2012;4(4):179-86. [ Links ]
18. Pereira da Silva CH, Vidigal GM, Jr., de Uzeda M, de Almeida Soares G. Influence of titanium surface roughness on attachment of Streptococcus sanguis: an in vitro study. Implant Dent. 2005;14(1):88-93. [ Links ]
19. Song F, Koo H, Ren D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J Dent Res. 2015;94(8):1027-34. [ Links ]
20. Hahnel S, Wieser A, Lang R, Rosentritt M. Biofilm formation on the surface of modern implant abutment materials. Clin Oral Implants Res. 2015;26(11):1297-301. [ Links ]
21. Peng TY, Lin DJ, Mine Y, Tasi CY, Li PJ, Shih YH, et al. Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers (Basel). 2021;13(6). [ Links ]
Conflict of Interest Statement The authors of this work declare that they have no conflicts of interest
Source of Funding This project did not receive funding from external sources other than the University of Concepción or the Master's program in Dental Sciences
Authorship and Collaboration Contribution Declaration: Study conception Data acquisition Data analysis Results discussion Manuscript drafting and editing Approval of the final version of the manuscript JF has contributed to a, b, c, e, f GSS has contributed to a, b, c, f LL has contributed to a, c, f MM has contributed to a, c, f MW has contributed to a, b, c, d, and f
Received: May 12, 2023; Accepted: March 12, 2024










 text in
text in