Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Revista Uruguaya de Cardiología
versión On-line ISSN 1688-0420
Rev.Urug.Cardiol. vol.26 no.1 Montevideo mar. 2011
ARTÍCULO DE REVISIÓN
Syncope: an overview of diagnosis and treatment
David G Benditt. MD, FACC, FHRS, FESC, FRCPC 1
1. Professor of Medicine, Co-Director Cardiac Arrhythmia Center, University of Minnesota Medical School. From the Cardiac Arrhythmia Center, Cardiovascular Division, Department of Medicine, University of Minnesota Medical School, MMC 508, 420 Delaware Street SE, Minneapolis, MN 55455, USA.
Correspondence: David G Benditt MD. Mail Code 508, 420 Delaware St SE. Minneapolis, MN, 55455
Email bendi001@umn.edu
INTRODUCTION
Syncope is a syndrome in which a relatively sudden-onset, brief loss of consciousness results from a temporary self-terminating period of total cerebral hypoperfusion (1-4). In this regard, it is important to note that other conditions (e.g., epilepsy, concussions, metabolic disturbances and intoxications) may also cause a temporary loss of consciousness (T-LOC) but nonetheless are not ‘syncope’ (3-5). Each of these differ from syncope either by the need for medical intervention to reverse the process (e.g., hypoglycemia) or by the underlying mechanism of the loss of consciousness (e.g., electrical disturbance in epilepsy, trauma in head injury, etc.) or both. Other conditions may also mimic syncope. These are often termed ‘syncope mimics’ or ‘pseudosyncope’, but differ from syncope inasmuch as they do not cause true loss of consciousness (e.g., conversion reactions, malingering, and cataplexy).
In itself, ‘syncope’ is not a complete diagnosis. Identifying the cause is important, since syncope may be a marker of increased mortality risk in some cases, but even more often may lead to physical injury resulting from falls or accidents, diminished quality-of-life, and possible restriction from employment or avocation. The goal should be to determine the cause of syncope with sufficient confidence to provide a reasonable assessment of prognosis, recurrence risk, and treatment options. The initial step is always the documentation of a comprehensive and detailed medical history (3-7).
LOSS OF CONSCIOUSNESS
Consciousness and ‘loss of consciousness’ (LOC) are complex concepts, but most physicians have a working understanding of what is meant (8). Essentially, LOC implies not only loss of awareness and appropriate responsiveness to external stimuli, but also loss of postural tone. Occasionally, however, symptoms may suggest that ‘syncope’ is imminent, but the full clinical picture does not evolve at that time; such cases are often termed ‘near-syncope’. In such instances, the patient may experience near loss of vision (‘grey-out’ due principally to transient loss of blood supply to the retina), diminution of hearing, and feeling ‘out-of-touch’ with their surroundings and/or confused. On the other hand, many times patients complain of less well defined symptoms such as “dizziness” or “lightheadedness”. These latter complaints (especially in the elderly) may be due to an ill-defined functional cerebral dysfunction triggered by transient hypotension (perhaps not severe enough to cause TLOC), but in most cases it is believed that such symptoms are not related to either ‘syncope’ or ‘near-syncope’, and should not be reported as such.
EPIDEMIOLOGY AND SOCIAL COST OF SYNCOPE
Syncope is known to be a relatively common cause of emergency department evaluation and hospital admission, but precise estimates of frequency are hard to establish, since in many reports the precision with which syncope has been differentiated from TLOC is unclear. Given this limitation, various reports estimate that syncope accounts for 1% to 3% of emergency department visits and 1% to 6% of hospital admissions (1,2).
An early report from the Framingham follow-up study (9) found that only 3,2% of adults admitted to one or more syncope spells. By contrast, in a more recent report from the same study (10) noted that 10% of 7814 subjects admitted to at least one syncope spell over a 17-year sampling time. In another extensive community-based study of American adults aged 45 years and older, Chen et al (2006) (11) reported that 19% of adults admitted to at least one syncope spell. Studies from Calgary (Canada), and Amsterdam (The Netherlands), reported similar results for estimates of community lifetime cumulative incidence. Ganzeboom et al (12) surveyed medical students and found that 39% had fainted at least once. The Calgary group (13) reported that by age 60 years 31% of males and 42% of females had fainted, very similar to the proportions reported by Amsterdam study (12,13). Thus, females were more likely to faint than males, or are at least more likely to volunteer the information. Taken together, the studies consistently suggest that 40% of people faint at least once in their lives with females perhaps being somewhat more susceptible. Further, within three years of the initial episode, about 35% of patients experience recurrences.
Recent estimates place the proportion of emergency room visits due to syncope at about 1% in Italy, France, and the United States (1,2). In the US, this translated into >1.127 million visits in 2006 based on ‘primary diagnoses’ recorded in the 2006 National Hospital Ambulatory Care survey, and >411,000 hospital admissions when syncope and collapse were listed among discharge diagnoses.
The direct cost of diagnosing and treating syncope is substantial. In the USA, an estimate of direct cost may be obtained from the Medicare database. In the year 2000 (20) the estimated total annual charges for syncope-related admissions were $5.4 billion, with a mean charge of $12,000 per hospitalization. Data in 2005-2006 from the United Kingdom provide estimates of £70 million per annum (14,15). On the other hand, determining ‘total cost’ is difficult since the indirect costs related to loss of earning by patients or family members are much harder to measure.
CLASSIFICATION OF THE CAUSES OF SYNCOPE
Syncope has many possible causes, and it is essential to approach the diagnostic possibilities in an organized manner (Figure 1). However, even after a thorough assessment, it may not be possible to assign a single cause for fainting. Patients often have multiple co-morbidities and as a consequence they may have several equally probable causes of fainting.
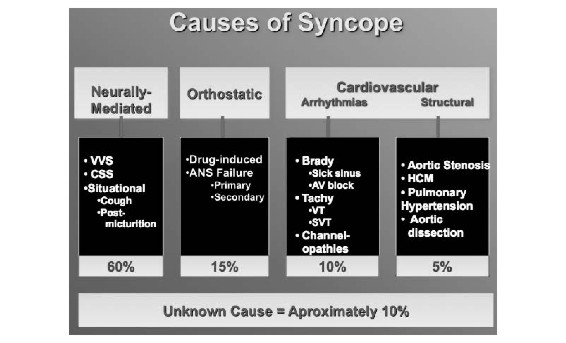
Figure 1. Classification of the principal causes of syncope. See text for details.
NEURALLY-MEDIATED REFLEX SYNCOPE SYNDROMES
Neurally-mediated reflex syncope refers to a group of related conditions in which symptomatic hypotension occurs as a result of neural reflex vasodilatation and/or bradycardia (1,2). The term ‘vasovagal syncope’ refers to a particular type of neurally-mediated reflex syncope also known as the ‘common faint’ (16). Vasovagal syncope has many manifestations and is generally considered to encompass faints triggered by emotional upset, fear, and pain, as well as those occurring in less well-defined circumstances. ‘Situational’ faints are essentially identical to ‘vasovagal faints’, but occur as the result of readily identified triggers (e.g., micturition, swallowing, etc). Carotid sinus syndrome (CSS) also falls into this category.
The neurally-mediated reflex faints, especially the vasovagal faint, are the most common causes of syncope overall, and are particularly prevalent in individuals without evidence of underlying heart or vascular disease (Table 1). The principal pathophysiological mechanism is the triggering of a neural reflex resulting in both hypotension due to vasodilation and an inappropriate chronotropic response (occasionally marked bradycardia or asystole) (17,18).

VASOVAGAL SYNCOPE (COMMON FAINT)
In susceptible individuals vasovagal syncope may be triggered by prolonged periods of upright posture, relative dehydration, excessively warm closed-in environments, or extreme emotions. Common places for these events are churches, restaurants and long queues. Warning symptoms may occur, and include feeling: hot or cold, sweaty, tachycardic, ‘short of air’, loss of hearing, nausea and change in breathing pattern. Physical findings often reported by bystanders (if the physician asks) in these cases include marked pallor, damp and cold (“clammy”) skin, and confusion. After the faint, if the patient is permitted to remain recumbent, recovery typically is very rapid, but a subsequent period of fatigue of variable duration is quite common.
Typically, the diagnosis is made from the medical history alone and no testing is needed. However, if the medical history does not provide sufficient basis to make the diagnosis, head-up tilt-table testing (HUT) may be helpful to support a diagnosis of vasovagal syncope (19,20). Such testing, in the absence of pharmacological provocation, has a specificity of approximately 90%. HUT is not known to be useful in the other neurally-mediated reflex faints.
CAROTID SINUS SYNDROME
Carotid sinus syndrome (CSS) and carotid sinus hypersensitivity (CSH) are two distinctly different entities. The first is a clinical syndrome resulting in syncope or near-syncope due to bradycardia and/or vasodilatation secondary to hypersensitivity of the carotid sinus barororeceptor. CSH, on the other hand, is the physiologic observation that may or may not have any clinical sequelae. If it is responsible for syncope, then the patient is diagnosed with CSS.
CSS is believed to be due to accidental manipulation of neck that results in external pressure on the carotid sinus baroreceptors. The susceptible individual (usually older male patients >65 years of age or individuals with previous neck surgery or irradiation) can often be demonstrated to exhibit carotid sinus hypersensitivity (CSH) during deliberate diagnostic carotid sinus massage applied by a suitably experienced physician (21-23).
The incidence of spontaneous carotid sinus syndrome (CSS) as a cause of faints has been thought to be relatively rare but recent studies from Newcastle UK, suggest that it may be responsible for falls in the elderly far more often than previously believed. Whether pacing can be an effective deterrent to ‘falls’ in the elderly is currently the subject of clinical trials such as PERF-CSH (Pacing in Elderly Recurrent Fallers with Carotid Sinus Hypersensitivity) (23).
SITUATIONAL SYNCOPE
Situational faints are diagnosed by their distinctive history, and it is usually unnecessary to evaluate these fainters in the clinical laboratory. As noted above, the pathophysiology of situational faints is similar to that of the conventional vasovagal faint except that the afferent trigger is identifiable. Thus, these faints include micturition syncope, deglutition or swallowing syncope, etc. Cough may also trigger reflex hypotension (24), although other non-reflex mechanisms for cough syncope have been proposed as well.
ORTHOSTATIC SYNCOPE
Orthostatic hypotension (OH) leading to syncope (orthostatic syncope), as its name implies, occurs as a result of a transient excessive cerebral hypotension that may occur when susceptible individuals arise from a lying or sitting to a standing position (25-27). Two basic forms are recognized. The first is so-called ‘immediate or initial hypotension’ and occurs almost immediately upon ‘active’ standing, and can be observed in young healthy individuals as well as in older patients. In fact, many healthy individuals experience a minor form of ‘immediate hypotension’ when they need to support themselves momentarily as they stand up. Essentially, in these instances, ‘immediate hypotension’ causes a transient self-limited ‘grey-out’. However, immediate hypotension may not always be benign; instability and falls are a risk in more frail individuals, and frank syncope can also occur. The second form of orthostatic hypotension is the classical form, otherwise termed the ‘delayed’ form. Symptoms usually occur several moments after standing up. The patient has already walked some distance, then collapses. The cause in both cases is deemed to be the failure of autonomic nervous system to respond to a sudden upright posture, but the delayed form tends not to reverse until gravity intervenes (i.e., the patient has fallen).
Either extrinsic factors or primary autonomic failure may account for orthostatic syncope. Extrinsic factors include dehydration from prolonged exposure to hot environments, inadequate fluid intake or excessive use of diuretics, anti-hypertensives or vasodilators. Chronic diseases such as diabetes, or peripheral neuropathy secondary to alcohol or other agents, may predispose patients to orthostatic syncope. Less commonly, orthostatic hypotension is the result of a primary autonomic diseases with inadequate reflex adaptations to upright posture (e.g., multisystem atrophy or Parkinson’s disease) (26,28).
CARDIAC ARRHYTHMIAS AS PRIMARY CAUSE OF SYNCOPE
Primary cardiac arrhythmias (i.e., those that are not secondary to neural-reflexes) are less commonly the cause of syncope than is either neurally-mediated reflex faints or orthostatic hypotension. However, given the propensity for arrhythmias to accompany other health conditions, and especially structural heart disease, the prognosis associated with arrhythmic syncope is of concern (albeit not usually due to the syncope per se, but more often as a result of the nature and severity of the underlying heart disease).
Cardiac tachyarrhythmias or bradyarrhythmias may be the primary cause of syncope if the abnormal heart rate (in conjunction with vascular compensatory responses) cannot maintain stable cerebral flow. Either or both may occur as a result of:
1) ‘intrinsic’ disease of the cardiac conduction system (e.g., intrinsic sinus node dysfunction or AV conduction system disease, or accessory conduction pathways), or
2) channelopathies (e.g., long QT syndrome, Brugada syndrome), or
3) structural cardiac or cardiopulmonary disease (i.e., structural cardiac or cardiopulmonary abnormalities, or
4) extrinsic effects such as drug-induced proarrhythmia.
Determining which, if any of the cardiac arrhythmias are responsible for syncope in a given individual can be difficult, especially if symptomatic events are infrequent. Electrophysiological testing may be helpful in some cases, but more often than not the findings are non-specific. Ambulatory ECG monitoring offers the opportunity to obtain symptom-arrhythmia concordance. However, 24-hour or 48-hour Holter type monitoring is not very effective because the likelihood that syncope will occur in that relatively brief time period is small. Longer-term monitoring (e.g., ECG ‘event’ recorders, mobile cardiac outpatient telemetry (MCOT) systems, implantable Loop recorders [ILRs]) increases the chance of finding a correlation. In particular, MCOT recorders and ILRs are especially valuable as they offer not only long recording periods, but also can detect and store events automatically (29-32).
SINUS NODE DYSFUNCTION
Symptoms in patients with sinus node dysfunction may be due to either brady- or tachyarrhythmias. Thus, while sinus/junctional bradycardia, sinus arrest or a sinus pause are common, paroxysmal atrial tachyarrhythmias fall into this category as well. In either case, the heart rate (HR) may be inadequate to support cerebral blood flow. In some patients both brady- and tachycardia may be contributory; for example, an abrupt spontaneous termination of an atrial tachycardia may be followed by long asystolic pause prior to recovery of a stable heart rate.
Electrophysiologic (EP) laboratory testing is of limited value in most patients with suspected sinus node dysfunction (33). Although measures such as sinus node recovery time (SNRT) and sinoatrial conduction time (SACT) exhibit a high level of specificity for sinus node disease, they are not very sensitive and do not provide essential symptom-arrhythmia concordance upon which to base treatment recommendations. MCOT and ILR recorders with direct rhythm-symptom correlation are better for this application. If, however, a correlation cannot be established despite reasonable effort, then it may be necessary to make a clinical treatment judgment based on other indirect evidence. Thus, severe sinus bradycardia while awake (< 40 beats per minute) and repetitive sinoatrial block or sinus pauses longer than 3 seconds may be used to justify pacemaker implantation. However, it is essential to continue clinical surveillance using the diagnostics within implanted devices to ascertain whether the correct course of action has been taken.
ATRIOVENTRICULAR CONDUCTION DISORDERS
Paroxysmal or persistent atrioventricular (AV) block can cause severe bradycardia and thereby lead to syncope (Figure 2). If a patient is found to have Mobitz type II second degree AV block, third degree AV block or alternating left and right bundle branch block, a causative diagnosis for the basis of syncope can be made with reasonable certainty. In any case, these diagnoses generally warrant pacemaker implantation. The American Heart Association/American College of Cardiology/Heart Rhythm Society pacemaker practice guidelines provide further details (34).
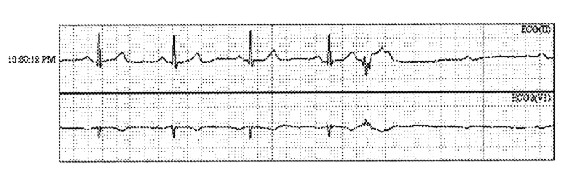
Figure 2. Paroxysmal AV block resulting in transient bradycardia in an older woman with recurrent syncope. A pacemaker was placed.
Other observations that may be suggestive of an AV conduction disorder being the cause of syncope, but at best provide only indirect evidence, include:
1) bifascicular block (left bundle branch block, right bundle branch bock with left anterior or left posterior fascicular block), and
2) Mobitz type I second degree AV block in older persons (usually defined as >70 years of age). In such cases further tests (such as MCOT and/or ILR monitoring, or EP testing) may be essential.
Once again, direct symptom-arrhythmia correlation by recording a spontaneous event is the best means of directing appropriate therapeutic intervention. However, a tentative diagnosis can be made in case of ventricular pauses > 3 seconds in duration when the patient is awake, or if Mobitz type II second degree or third degree (i.e., complete or ‘high grade’) AV block is discovered. In the setting of nothing more than bifascicular block or non-specific intra-ventricular conduction delay, invasive EP study may be helpful.
In the EP laboratory, the cardiac conduction system can be ‘stressed’ by pacing and / or drug infusions (e.g., procainamide, ajamline) with the objective of unmaking susceptibility to higher levels of conduction block that might induce syncope. Thus, a typical study strategy would incorporate measurement of the infra-His conduction time (H-V interval), incremental atrial pacing to ascertain the conduction capability of the AV node-His-Purkinje system, and if needed provocative testing with direct acting intravenously administered antiarrhythmic drugs, such as ajmaline (not available in USA) or procainamide.
EP laboratory findings can generally only provide a presumptive basis for syncope. Thus, if the H-V interval is greater than 100ms, or if incremental atrial pacing produces second or third degree AV block at heart rates £120 beats/minute, or if high degree AV block is observed at relatively slow paced rates (typically £120/minute) after drug provocation, a basis for symptoms may be presumed (35,36). However, although a pacemaker may be justified, further monitoring to ascertain whether future syncope is prevented is essential; consequently, it is best to use an implanted device with comprehensive monitoring capability.
SUPRAVENTRICULAR AND VENTRICULAR TACHYARRHYTHMIAS
Supraventricular tachycardia (SVT) and ventricular tachyarrhythmias (VT) account for a minority of syncope cases, but are important given their potential for cure by ablation in the case of SVT, and concern regarding prognosis in VT patients.
The diagnostic evaluation of patients with suspected SVT or VT, in the absence of ‘hard’ ECG evidence during ambulatory ECG monitoring, usually entails EP testing. In the case of SVTs, reproducible induction of tachycardia can be relied upon as likely unmasking the cause and the therapeutic options in that case usually focus on ablation. Similarly, SVT or atrial fibrillation in the setting of preexcitation syndrome (i.e., WPW syndrome) is usually sufficient to warrant accessory connection catheter ablation.
In the case of VT, EP study is of value mainly in the subset of patients with ischemic heart disease. In other scenarios (see below) EP testing is usually not considered to be helpful, or at best its use is controversial. Further, even in the setting of ischemic disease, the potential for induction of non-specific tachyarrhythmias in the EP laboratory is a problem. Given that limitation, it would be helpful to have effective non-invasive ‘risk stratification’ tools in order to improve the likelihood that an invasive study will be productive, but these not yet available. Tests that have at various times been advocated to risk stratify patients include signal averaged ECG (SAECG), heart rate variability (HRV) and micro-volt T wave alternans. Unfortunately, experience indicates that while these tests have a reasonable ‘negative predictive value’, they exhibit only a low positive predictive value.
Ventricular tachyarrhythmias (VT) tend to be more closely associated with syncope than are SVTs, particularly if structural heart disease is present. Thus, VT occurring in the setting of ischemic heart disease, valvular heart disease or certain dilated cardiomyopathies may result in symptomatic hypotension. Similarly, VT in obstructive cardiomyopathies and arrhythmogenic right ventricular dysplasia / cardiomyopathy (ARVD/C) may cause syncope. However, VT also occurs in some individuals with important examples are long-QT syndrome, and Brugada syndrome.
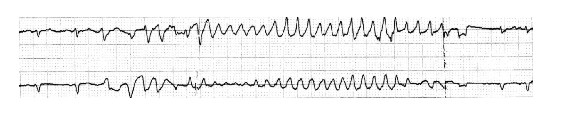
In terms of presenting with syncope, torsade de pointes ventricular tachycardia is a particularly important form of VT to keep in mind (Figure 3). This arrhythmia is a characteristic feature of the long-QT syndromes, may be very fast and is often it is self-terminating. Consequently syncope may occur (37). However, on occasion progression of the arrhythmia to ventricular fibrillation can happen; this emphasizes the importance of not overlooking these diagnoses.
Figure 3. Example of no-sustained Torsade de Pointes VT in a patient with long QT syndrome and syncope.
Non-sustained VT (NSVT) is a common finding during evaluation of syncope patients (whether by Holter or by extended-duration AECG monitoring), but is diagnostically a much less specific finding than is sustained VT. The isolated presence of NSVT, particularly in the absence of evident structural cardiac disease or ECG evidence of a ‘channelopathy’ should not typically be considered a ‘causative’ diagnosis. On the other hand, if monomorphic sustained VT is reproducibly inducible during EP study, a basis for syncope seems likely. In such instances, therapy (drugs, ablation) directed at the arrhythmia is appropriate. Implantable device prophylaxis (e.g., implantable cardioverter-defibrillator, ICD) may also be warranted. However, while ICDs are helpful to prevent sudden arrhythmic death, they may not prevent episodes of dizziness or syncope, which may occur at the onset of the abnormal rhythm before the device is activated.
CARDIOVASCULAR AND CARDIOPULMONARY DISEASE
Syncope may occur as a direct result of severe underlying cardiac or cardiopulmonary disease. The most common causes are acute coronary syndromes (ACS), and acute myocardial infarction. Others, although less common, are severe aortic stenosis or hypertrophic obstructive cardiomyopathy (HOCM), acute aortic dissection, and severe pulmonary hypertension. In each of these, despite the potential for important hemodynamic consequences of the conditions, it is generally believed that the syncope is due to a neurally-mediated reflex, rather than a direct consequence of disturbed hemodynamics.
CEREBROVASCULAR DISEASE AND RELATED CONDITIONS
Cerebrovascular disease and related conditions (with the possible exception of syncope associated with migraine) are very rare causes of syncope. Evaluation of these conditions (e.g., vertebrobasilar transient ischemic attacks, subclavian steal syndrome) should be reserved to those few instances in which there is strong medical history evidence favoring the possibility. Otherwise, much energy and cost will be expended with low diagnostic yield.
Migraines are probably the most important condition in this group of causes of syncope (58,59). However, as a rule, migraines do not cause syncope directly. Evidence suggests that migraine may trigger a neurally-mediated vasovagal reflex syncope in most cases.
SYNCOPE MIMICS AND PSEUDO-SYNCOPE
There are two important groups of conditions which may present with real or apparent T-LOC but that should not be considered as ‘syncope’. First of this group are conditions which cause true TLOC, but their pathophysiological mechanism differs from true syncope. These may be considered as ‘Non-syncope TLOC’, with epilepsy being the most important example, but trauma leading to concussion (such as might occur after an accidental fall in an older patient) must be kept in mind as well. The second group of conditions comprises those in which consciousness is never really lost. These are best termed ‘Pseudosyncope’ (neurologists may use the term ‘pseudoseizure’). The most important cause is psychiatric conversion disorders. Malingering might also present as pseudosyncope, but this seems to be rare. Since these two groups of conditions are not ‘true’ syncope, they are not discussed further here. However, ‘drop attacks’ warrant additional mention.
‘Drop attacks’ belong in the ‘pseudo-syncope’ group. In ‘classical’ description, the ‘drop attack’ is an uncommon condition in which postural tone is lost abruptly and patient (most often females) falls to the ground. The history-taker can determine by careful questioning of the patient’s recollection during the attack whether consciousness was retained. Nevertheless, in some cases the patient may actually have lost consciousness and simply has no recollection of having done so. Consequently, in some individuals these episodes can be mistaken for syncope, whereas in others they may actually have been a ‘true’ syncope. Establishing a certain diagnosis is challenging and may be frustrating.
Clinical experience suggests that many patients with pseudo-syncope often have a history of ‘true’ syncope as well. Perhaps, the rare true faints lead to psychiatric- or stress-related responses that ultimately result in an increasingly frequent series of ‘syncope-like’ episodes (i.e., ‘pseudosyncope’).
ESTABLISHING THE CAUSE OF SYNCOPE
INITIAL EVALUATION OF THE PATIENT WITH SUSPECTED SYNCOPE
The evaluation of patients with suspected syncope begins with a careful history taking (Table 2). However, obtaining a reliable history may be a problem. Elderly patients and those with cognitive impairment may not be able to remember all events, while other patients may not volunteer a complete history due to risk of losing their job, or driving privileges. Consequently, witnesses to symptomatic events should be included in the history-taking process, whenever possible.
In brief, it is essential that as many symptom events be assessed in detail. The preceding circumstances, premonitory symptoms, and subsequent outcome should be documented for as many episodes as possible. If a pattern emerges, the diagnosis may become evident without the need for further testing. Similarly, careful note should be made of patient’s co-morbidities (for example diabetic neuropathy, autonomic dysfunction). A pre-prepared patient questionnaire may prove helpful to save time and still acquire the needed details.
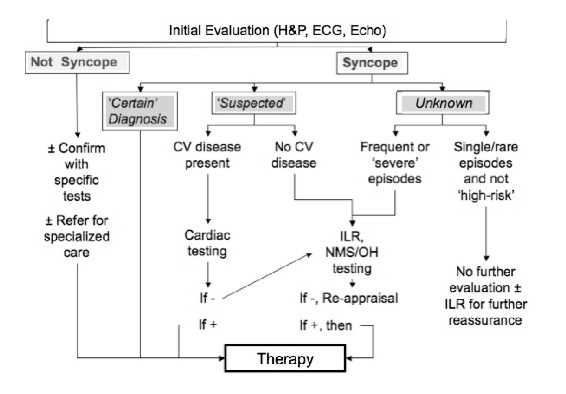
Based on the initial evaluation, it should be possible to classify patients into one of three categories: Certain diagnosis, Suspected diagnosis or Unknown diagnosis. Thereafter, the diagnostic flow can reasonably follow the strategy depicted in Figure 4 which has been modified from that devised by the European Society of Cardiology Syncope Guidelines Task Force (2). The goal is to choose subsequent diagnostic tests in a careful manner to maximize cost-effectiveness and minimize the number of studies.
Figure 4. Diagnostic flow of Syncope (adapted from reference 2)
SHOULD THE DIAGNOSIS BE PURSUED IN HOSPITAL OR IN THE OUTPATIENT SETTING?
In general, the driving force determining whether the patient with presumed syncope should be hospitalized for diagnostic evaluation and if necessary for treatment initiation, is most often concern regarding the individual’s immediate mortality risk. Secondary issues include potential for physical injury (e.g., falls risk) and to a lesser extent whether certain treatments inherently require hospital monitoring for safe initiation. Thus, for example, patients with syncope accompanying complete heart block, ventricular tachycardia, acute aortic dissection, or pulmonary embolism, should be admitted to the hospital and preferably to an ECG monitored unit. On the other hand, most vasovagal fainters can be sent home after careful discussion of the nature of the problem and simple preventative maneuvers (e.g., hydration, avoidance of hot crowded environments, etc). Clinic follow-up suffices in most of these cases.
For syncope patients in whom the etiology remains unknown after the initial emergency department or ambulatory clinic evaluation, the need for hospitalization is less well defined and consequently so-called “risk stratification” methods have been advocated (38-45). In essence, the goal is to ascertain the relative risk for adverse outcome using patients’ clinical features and presenting characteristics. Based on this assessment one attempts to determine if hospitalization is prudent.
The risk stratification methods differ in various published studies. Nonetheless, the Syncope Evaluation in the Emergency Department Study (SEEDS), The Osservatorio Epidemiologico sula Sincope nel Lazio (OESIL) study, The San Francisco Syncope Rule (SFSR) study, and the European Society of Cardiology and American College of Emergency Physicians (ACEP) guidelines (40-45) each uses clinical data that is readily accessible to the ED physician or general practitioner. These data include symptoms, signs, basic laboratory results and clinical experience (‘judgment’).
At present, it is not possible to determine whether one or other risk stratification scheme is superior to the others. Head-to-head testing is needed. In this regard, the Risk Stratification Of Syncope in the Emergency Department (ROSE) study compared the performance of OESIL score, and the SFSR recommendations with emergency department guidelines of a single center in United Kingdom (Royal Infirmary of Edinburgh) (44). The latter institution used a guideline based on ESC, American College of Physicians and ACEP guidelines. The goal was to determine which of these Risk Stratification tools best predicted short-term (1 week and 1 month) and medium-term (3 months) serious outcomes for patients presenting with syncope. In this regard, each of the scores was able to identify an increased probability of medium-term serious outcome in patients with syncope. The SFSR showed good sensitivity at the expense of an increased frequency of admission to the hospital. On the other hand, the Royal Infirmary’s center’s own guideline, and the OESIL score, was not sufficiently sensitive to be able to reduce admissions without missing patients at risk of serious outcome.
ROLE OF SYNCOPE MANAGEMENT UNITS (SMUS)
An as yet incompletely answered question is whether “SMUs” can help solve the problem of too many low- and intermediate-risk syncope patients being admitted to hospital where they often are submitted to unneeded expensive diagnostic tests as pointed out in the EGSYS (The Evaluation of Guidelines in Syncope Study) reports (69). In this regard, 2 recent prospective observational studies demonstrated improved syncope management in the hospital by using guideline-based decision making software and a team of specially-trained personnel in a SMU. In the SEEDS study (46), 103 patients were randomized to ‘standard care’ or SMU after initial assessment. The study found that a presumptive diagnosis of the cause of syncope was significantly increased from 10% in the ‘standard care’ patients to 67% among those who underwent SMU evaluation; hospital admission was reduced from 98% among the ‘standard care’ patients to 43% among the SMU patients. Similarly, the total length of patient-hospital days was reduced by >50% for patients in the SMU group.
The potential for the ESC Guidelines to facilitate management of syncope patients referred to ED’s of 11 Italian general hospitals was investigated in EGSYS-2 (46), and facilitated by use of purpose-designed software in addition to personnel training at test sites. A definite diagnosis was established in 98% of cases, with the vast majority being either neurally-mediated reflex or orthostatic faints. The initial evaluation (history, physical examination, and electrocardiogram) established a diagnosis in 50% of cases. The investigators further compared the outcomes of 745 patients managed with this “standardized care” system to 929 patients managed with usual care. In the group designated to “standardized-care”, hospitalizations were fewer, in-hospital stay was shorter, fewer tests were performed per patient, and cost per patient and mean cost per diagnosis were lower.
TREATMENT
Treatment of the syncope patient may be divided into 2 parts. The first is management of an acute syncopal event. Although physicians are only infrequently involved in this aspect of care, treatment of the acute episode requires protection of the patient from injury, assuring that the victim is placed safely in a gravitationally neutral position, and documenting adequacy of respiration and circulation. Thereafter, recovery is spontaneous. The second part is prevention of syncope recurrences.
NEURALLY-MEDIATED REFLEX FAINTS
In most cases, vasovagal syncope or situational faints are solitary or at most very infrequent events. Therefore, most patients need little more than reassurance and education about the nature of this condition and the types of circumstances that might increase their risk of recurrence (e.g., prolonged standing, warm environments, etc.). However, in certain individuals, the faints are more frequent for at least a period of time during their lives. In these cases, should also be taught to be alert to warning symptoms such as feeling of being hot or cold, sweaty, clammy, short of breath, or nauseated. The goal is to educate susceptible individuals to recognize impending events and take action in order to prevent the episodes (e.g., sitting or lying down with feet elevated). Similarly, if a patient knows that sight of blood could bring about a vasovagal faint, he/she may either avoid the situation or possibly be preconditioned to perform maneuvers to prevent the faint (e.g., aggressive hydration, arm-tensing, leg crossing).
With regard to techniques for acute intervention to abort an imminent vasovagal or situational faints, patients who have warning symptoms should be educated about specific physical counter-maneuvers (PCM); thus, squatting, arm-tensing, leg-crossing, and leg-crossing with lower body muscle tensing have proved useful for averting an abrupt vasovagal reaction (47-50). In the recent Physical Counterpressure Manoeuvres Trial (PC-Trial), van Dijk et al. demonstrated that physical counterpressure maneuvers (PCM) can reduce the total burden and recurrence rate of syncopal events (50). Consequently, PCM education should be part of the treatment strategy, especially in patients with warning symptoms prior to their faints.
Longer-term prevention of vasovagal syncope recurrences focuses on use of fluids rich in electrolytes and increased dietary salts. Examples would include ‘sport drinks’ although those with lowest carbohydrate content are preferred. Highly motivated patients could also be provided with instructions regarding upright standing training (so-called tilt-training). ‘Tilt training’ involves progressively lengthening periods of enforced upright posture, the goal of which is to improve tolerance to upright posture (51). In addition, a number of pharmacologic approaches have been proposed for vasovagal syncope. However, most of these have not been studied in randomized, controlled trials. Examples of such medications include fluorocortisone, beta-adrenergic blocking drugs, and vasoconstrictor agents like midodrine and serotonin re-uptake inhibitors. Although these may work well in individual patients, only midodrine has shown effectiveness in controlled studies (52-54).
Cardiac pacing has received considerable study as a potential treatment option in patients with very frequent vasovagal faints. However, despite early favorable reports, pacing is not considered very effective for preventing syncope in most patients. On the other hand, the ISSUE-2 trial indicated that if marked bradycardia during spontaneous syncope has been documented by ILR recording, then pacing may be warranted in recurrent fainters (30). ISSUE-2 was an observational study. ISSUE-3, which just concluded enrollment in November 2010, is reassessing this issue in a randomized controlled study.
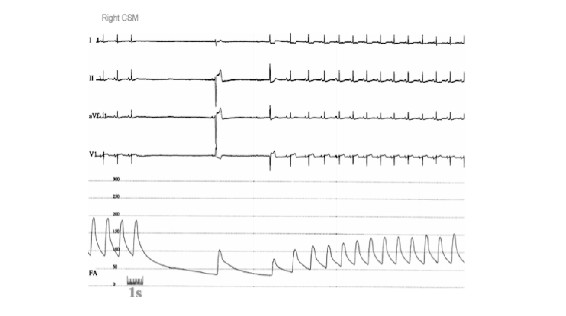
Carotid sinus syndrome (CSS) is a special form of neurally-mediated reflex syncope that tends to occur in older individuals. Falls and injury is a real concern in these patients if untreated (23,55). In CSS, despite the presence of both cardioinhibitory and vasodepressor features, pacing appears to be highly effective for preventing recurrences (Figure 5).
Figure 5. Recording of ECG leads !, II, aVL, and V1, and arterial pressure during carotid sinus massage (CSM) in an older male with syncope and suspected carotid sinus syndrome. Note that despite recovery of the heart rate after the initial asystolic period, the blood pressure remains well below baseline value. This observation (i.e., the persistent hypotension) is supportive of a ‘vasodepressor’ component to the CSM response in addition to the induced bradycardia (also known as the cardioinhibitory aspect of the CSM response).
ORTHOSTATIC SYNCOPE
Treatment of orthostatic syncope parallels in many respects the strategy discussed earlier for vasovagal and situational faints. The principal differences are: 1) the duration of treatment is likely to be longer, 2) affected individuals are typically older and more frail making physical maneuvers more difficult to employ, and 3) patients are more prone to supine hypertension thereby complicating the overall treatment strategy.
In many cases, syncope associated with postural change may be caused by low circulating plasma volume, or inadequate vascular constriction upon moving to the upright posture (often drug-induced), or both. Consequently, one of the basic tenets of treatment is expanding central circulating volume. Additionally, affected individuals should be instructed to try and avoid certain predisposing conditions (e.g., prolonged exposure to hot environments) or medications that decrease volume status (e.g., diuretics) or that impair vasoconstriction (e.g., vasodilators, beta-adrenergic blockers). In some severe cases, use of vasoconstrictors (like midodrine) or volume expanders (e.g., fludrocortisone) may be necessary to maintain adequate cerebral perfusion. Finally, patients who exhibit poor autonomic function may benefit from tilt-training (discussed earlier), counter-pressure clothing such as fitted stockings and abdominal compression devices. In patients with severe pure autonomic failure, bolus water intake, especially before arising from bed in the morning, may result in a substantial and sustained increase in blood pressure (56).
PRIMARY CARDIAC ARRHYTHMIAS
The treatment of cardiac arrhythmias causing syncope is determined by the specific arrhythmia that is deemed to be at fault. Given the numerous possibilities and the many factors that go into selecting appropriate therapies for these arrhythmias, only a very brief overview is provided here.
As noted earlier, sinus node disease may cause syncope either due to bradycardia or tachycardia mechanisms as discussed earlier. When a temporal correlation between syncope and bradycardia has been established, pacemaker implantation is the treatment of choice. Dual-chamber or atrial pacing is preferred. In the case of a tachyarrhythmia-induced syncope, both antiarrhytmic drugs and ablation are reasonable treatment considerations. The final choice depends on specific clinical circumstances and patient preferences.
Treatment of AV conduction system disease in syncope patients does not differ measurably from their treatment in other patients. However, once again it is crucial to obtain concordance between syncopal episodes and bradycardia. If such a correlation is found and the cause of AV block is irreversible, pacemaker implantation is a class I indication.
In general, first degree AV block and Type I second degree AV block (Wenkebach type) with a narrow QRS do not cause syncope and are not indications for a pacemaker. However, when second degree Type I block occurs in the setting of evident infra-nodal conduction system disease (e.g., wide QRS), or in older individuals (>70 years of age) pacemaker therapy is indicated. Similarly patients who have Mobitz type II AV block carry a high risk for intermittent high grade AV block causing syncope, and are candidates for pacing therapy. On the other hand, these same patients often exhibit substantial structural heart disease and therefore, and are also prone to ventricular tachyarrhythmias. Management in such cases may be better accomplished by ICD therapy.
When reentrant paroxysmal supraventricular tachyarrhythmias are deemed to be the cause of syncope transcatheter ablation is the treatment of choice in most cases. Drug therapy remains an option, however, given the high success rate with ablation and its ready availability, the ablation track seems more desirable. Similarly, most atrial tachyarrhythmias are now subject to cure by transcatheter ablation technique. This certainly is true for atrial flutter, most ectopic atrial tachyacrdias and increasingly for paroxysmal atrial fibrillation. However, drug therapy remains the most commonly used approach by most physicians. In this regard, drugs that reduce heart rate in tachycardia may be sufficient to prevent syncope. The most common of these agents are: beta-adrenergic blockers, and calcium channel blockers. If the desire is also to try and suppress tachycardia recurrences, a membrane active antiarrhythmic drug is also needed. Finally, in the case of refractory symptomatic atrial tachyarrhythmias (particularly atrial fibrillation) with rapid ventricular rates, treatment by His Bundle ablation and placement of a permanent pacemaker has proved to be safe and highly effective (57).
The treatment of ventricular tachyarrhythmias in syncope patients depnds on the clinical circumstance. Patients with ischemic heart disease or dilated cardiomyopathies and severely diminished left ventricular function (i.e., LV ejection fractions <35%) have a high mortality rate and benefit from ICD therapy. As alluded to earlier, whether syncope will be prevented by ICD treatment is less certain since the devices must take time before intervening with pacing or shock treatment (58). Consequently, in the syncope patient concomitant antiarrhythmic drug therapy and/or ablation may be needed.
Ventricular tachyarrhythmias arising from the Right Ventricular Outflow Tract (RVOT) or Left Ventricular Outflow Tract (LVOT) or the intraventricular fascicular system or bundle-branch system (i.e., bundle-branch reentry) are infrequent causes of syncope. However, when they are responsible for symptoms, transcatheter ablation is probably the treatment of choice. In arrhythmogenic right ventricular dysplasia (ARVD), treatment strategies are controversial. Medical treatment has not been found to be very effective. Furthermore, since there are potentially many regions of the heart that are affected, long-term efficacy of transcatheter ablation is limited. Therefore, syncope patients with ARVD, in whom ventricular tachyarrhythmias have been documented, may be best served by placement of an ICD. Once again, however, syncope may not necessarily be completely averted and concomitant medications may be needed.
Long QT syndrome, Brugada syndrome, and other ‘channelopathies’ are increasingly recognized as causes of syncope due to polymorphous VT (so-called ‘torsades de pointes’) (Figure 4). If there is no evidence for a reversible problem, the use of ICD therapy may be essential to prevent further syncope and also reduce sudden death risk.
STRUCTURAL CARDIOVASCULAR OR CARDIOPULMONARY DISEASE
In these conditions, syncope may be only one subset of symptoms being experienced by the patient. Correction of the structural lesion or its consequence is usually the treatment of choice. As an example, patients with valvular aortic stenosis, pericardial disease, atrial myxoma and congenital cardiac anomaly benefit from a direct corrective approach. In other cases, such as primary pulmonary hypertension or restrictive cardiomyopathy, the structural lesions are not correctable. In the case of HOCM, modification of the outflow gradients surgically may be accompanied by substantial risk and morbidity, and medical therapy is not very effective. If it is clear that syncope is due to the obstruction (as opposed to arrhythmias discussed earlier) cardiac pacing to diminish dynamic outflow gradients and transcatheter alcohol septal ablation may prove helpful.
CEREBROVASCULAR
As noted previously, cerebrovascular disease is rarely the cause of syncope. Migraines may be the most important consideration. The basis for the faints in migraineurs is unclear, but there is an established relationship between migraines and autonomic dysfunction (59). In any case, the medical management includes use of â-blockers and cranial/basilar artery vasoconstrictors such as sumatriptan (60).
Strokes and carotid system transient ischemic attacks (TIA) are almost never the cause of syncope. On the other hand, vertebrobasilar TIAs are a possible but extremely rare cause of faints. Treatment includes anti-platelet agents or anti-coagulation. When diagnoses in this category are suspected, neurological or neurosurgical consultation should be sought.
Subclavian steal syndrome is another, but also very rare cause of syncope in this category. Its treatment requires intervention, either surgically or by catheter based angioplasty (61).
SYNCOPE MIMICS
The ‘syncope mimics’ include serious medical conditions like epilepsy, diabetic coma, intoxication, severe hypoxia or hypercapnia. While these conditions require urgent medical attention, they are not the cause of true syncope (see earlier discussion). Among the conditions that are defined as syncope mimics, psychogenic pseudo-syncope often accompanied by anxiety attacks and hyperventilation is among the most common, and is very difficult to treat. In these patients it is very easy for health care providers to overlook serious underlying health issues. Only when one is convinced that there is no clinically significant cardiac or pulmonary problem should subsequent care be directed toward psychiatric consultation, and biofeedback.
CONCLUSIONS
Syncope is a form of transient loss of consciousness (TLOC) that is self-limited and reversible due to transient spontaneously reversible cerebral hypoperfusion. Delineating the underlying causes and the risk of adverse outcome may be challenging. However, careful assessment is important as syncope tends to recur; physical injury resulting from falls or accidents, diminished quality-of-life, and possible restriction from employment or avocation are real concerns. Determining that certain individuals are at ‘low mortality risk’ is insufficient. The goal in every case should be to determine the cause of syncope with sufficient confidence to provide patients and family members with a reliable assessment of prognosis, recurrence risk, and treatment options.
REFERENCES
1. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines on management (diagnosis and treatment) of syncope – 2009. The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Guidelines for the diagnosis and management of syncope (version 2009). European Heart J 2009; 30: 2631-71.
2. Brignole M, Alboni P, Benditt D, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, et al. Guidelines on management (diagnosis and treatment) of syncope, update 2004. Europace 2004; 6: 467-537.
3. Wieling W, Thijs RD, van Dijk N, Wilde AA, Benditt DG. van Dijk JG. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain 2009; 132: 2630-42.
4. Benditt DG, Nguyen JT. Syncope: Therapeutic approaches. J Am Coll Cardiol 2009; 53(19): 1741-51.
5. van Dijk JG, Thijs RD, Benditt DG,Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nature Rev Medicine 2009; 5: 438-48.
6. Alboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37: 1921-8.
7. Colman N, Nahm K, van Dijk JG, Reitsma JB, Wieling W, Kaufmann H. Diagnostic value of history taking in reflex syncope. Clin Auton Res 2004; 14 Suppl 1: 37-44.
8. Posner JB, Plum F, R2 Library. Plum and posner’s diagnosis of stupor and coma. Oxford: Oxford University Press; 2007: 71.
9. Savage DD, Corwin L, McGee DL, Kannel WB, Wolf PA. Epidemiologic features of isolated syncope: The Framingham study. Stroke 1985; 16: 626-9.
10. Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347: 878-85.
11. Chen LY, Shen WK, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Prevalence of syncope in a population aged more than 45 years. Am J Med 2006; 119: 1088.e1-1088.e7.
12. Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol 2003; 91: 1006-8, A8.
13. Serletis A, Rose S, Sheldon AG, Sheldon RS. Vasovagal syncope in medical students and their first-degree relatives. Eur Heart J 2006; 27: 1965-70.
14. Sun BC, Emond JA, Camargo CA Jr. Characteristics and admission patterns of patients presenting with syncope to US emergency departments, 1992-2000. Acad Emerg Med 2004; 11: 1029-34.
15. Sun BC, Emond J, Comargo C Jr. Direct medical costs of syncope=related hospitalizations in the United States. Am J Cardiol 2005; 95: 668-71.
16. Thijs RD, Benditt DG, Mathias CJ, Schondorf R, Sutton R, Wieling W, et al. Unconscious confusion—a literature search for definitions of syncope and related disorders. Clin Auton Res 2005; 15: 35-9.
17. van Lieshout JJ, Wieling W, Karemaker JM, Eckberg DL. The vasovagal response. Clin Sci (Lond) 1991; 81: 575-86.
18. Wieling W. Maintaining blood pressure whilst upright: Physiology and potential for disturbances to cause syncope. In: Benditt DG, European Society of Cardiology, eds. The Evaluation and Treatment of Syncope : A Handbook for Clinical Practice. 2nd ed. Malden, Mass.: Blackwell Pub.; 2006:301.
19. Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, Lerman BB, et al. Tilt table testing for assessing syncope. American College of Cardiology. J Am Coll Cardiol 1996; 28: 263-75.
20. Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: A useful test for investigating unexplained syncope. Lancet 1986; 1: 1352-5.
21. Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Neurally mediated syncope detected by carotid sinus massage and head-up tilt test in sick sinus syndrome. Am J Cardiol 1991; 68: 1032-6.
22. Morley CA, Sutton R. Carotid sinus syncope. Int J Cardiol 1984; 6: 287-93.
23. Parry SW, Steen N, Bexton R, Tynan M, Kenny RA. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity (PERF-CSH): A randomized, double-blind, placebo controlled cross-over trial. Heart 2009; 95(5): 405-9.
24. Benditt DG, Samniah N, Pham S, Sakaguchi S, Lu F, Lurie KG, et al. Effect of cough on heart rate and blood pressure in patients with “cough syncope”. Heart Rhythm 2005; 2: 807-13.
25. Bannister R. Chronic autonomic failure with postural hypotension. Lancet 1979; 2: 404-6.
26. Hopkins A, Neville B, Bannister R. Autonomic neuropathy of acute onset. Lancet 1974; 1: 769-71.
27. Low PA, Opfer-Gehrking TL, McPhee BR, Fealey RD, Benarroch EE, Willner CL, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc 1995; 70: 617-22.
28. Koike Y, Takahashi A. Autonomic dysfunction in parkinson’s disease. Eur Neurol 1997; 38 Suppl 2: 8-12.
29. Brignole M, Menozzi C, Moya A, Garcia-Civera R, Mont L, Alvarez M, et al. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104: 2045-50.
30. Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Moya A, Wieling W, et al. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27: 1085-92.
31. Menozzi C, Brignole M, Garcia-Civera R, Moya A, Botto G, Tercedor L, et al. Mechanism of syncope in patients with heart disease and negative electrophysiologic test. Circulation 2002; 105: 2741-45.
32. Moya A, Brignole M, Menozzi C, Garcia-Civera R, Tognarini S, Mont L, et al. Mechanism of syncope in patients with isolated syncope and in patients with tilt-positive syncope. Circulation 2001; 104: 1261-7.
33. Benditt DG, Gornick CC, Dunbar D, Almquist A, Pool-Schneider S. Indications for electrophysiologic testing in the diagnosis and assessment of sinus node dysfunction. Circulation 1987; 75: III 93-102.
34. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008; 117: e350-408.
35. Dhingra RC, Palileo E, Strasberg B, Swiryn S, Bauernfeind RA, Wyndham CR, et al. Significance of the HV interval in 517 patients with chronic bifascicular block. Circulation 1981; 64: 1265-71.
36. Scheinman MM, Peters RW, Suavé MJ, Desai J, Abbott JA, Cogan J, et al. Value of the H-Q interval in patients with bundle branch block and the role of prophylactic permanent pacing. Am J Cardiol 1982; 50: 1316-22.
37. Jons C, Moss AJ, Goldenberg I, Liu J, McNitt S, Zareba W, et al. Risk of fatal arrhythmic events in long QT syndrome after syncope. J Am Coll Cardiol 2010; 55: 783-8.
38. Huff JS, Decker WW, Quinn JV, Perron AD, Napoli AM, Peeters S, et al. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with syncope. Ann Emerg Med 2007; 49: 431-44.
39. Can I, Benditt DG. Syncope: To admit or not to admit. J Roy Coll Phys Edinburgh 2009; 39: 236-42.
40. Shen WK, Decker WW, Smars PA, Goyal DG, Walker AE, Hodge DO, et al. Syncope evaluation in the emergency department study (SEEDS): A multidisciplinary approach to syncope management. Circulation 2004; 110: 3636-45.
41. Ammirati F, Colivicchi F, Minardi G, De Lio L, Terranova A, Scaffidi G, et al. The management of syncope in the hospital: The OESIL study (osservatorio epidemiologico della sincope nel lazio). G Ital Cardiol 1999; 29: 533-9.
42. Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M, et al. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: The OESIL risk score. Eur Heart J 2003; 24: 811-9.
43. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco syncope rule to predict patients with short-term serious outcomes. Ann Emerg Med 2004; 43: 224-32.
44. Reed MJ, Newby DE, Coull AJ, Jacques KG, Prescott RJ, Gray AJ. The risk stratification of syncope in the emergency department (ROSE) pilot study: A comparison of existing syncope guidelines. Emerg Med J 2007; 24: 270-5.
45. Bartoletti A, Fabiani P, Adriani P, Baccetti F, Bagnoli L, Buffini G, et al. Hospital admission of patients referred to the emergency department for syncope: A single-hospital prospective study based on the application of the european society of cardiology guidelines on syncope. Eur Heart J 2006; 27: 83-8.
46. Brignole M, Ungar A, Bartoletti A, Ponassi I, Lagi A, Mussi C, et al. Standardized-care pathway vs. usual management of syncope patients presenting as emergencies at general hospitals. Europace 2006; 8: 644-50.
47. Brignole M, Croci F, Menozzi C, Solano A, Donateo P, Oddone D, et al. Isometric arm counter-pressure maneuvers to abort impending vasovagal syncope. J Am Coll Cardiol 2002; 40: 2053-9.
48. Krediet CT, van Dijk N, Linzer M, van Lieshout JJ, Wieling W. Management of vasovagal syncope: Controlling or aborting faints by leg crossing and muscle tensing. Circulation 2002; 106: 1684-9.
49. Krediet CT, de Bruin IG, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol 2005; 99: 1697-703.
50. van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, et al. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: The physical counterpressure manoeuvres trial (PC-trial). J Am Coll Cardiol 2006; 48: 1652-7.
51. Ector H, Reybrouck T, Heidbuchel H, Gewillig M, Van de Werf F. Tilt training: A new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pacing Clin Electrophysiol 1998; 21: 193-6.
52. Grubb BP, Samoil D, Kosinski D, Temesy-Armos P, Akpunonu B. The use of serotonin reuptake inhibitors for the treatment of recurrent syncope due to carotid sinus hypersensitivity unresponsive to dual chamber cardiac pacing. Pacing Clin Electrophysiol 1994; 17: 1434-6.
53. Grubb BP, Wolfe DA, Samoil D, Temesy-Armos P, Hahn H, Elliott L. Usefulness of fluoxetine hydrochloride for prevention of resistant upright tilt induced syncope. Pacing Clin Electrophysiol 1993; 16: 458-64.
54. Sra J, Maglio C, Biehl M, Dhala A, Blanck Z, Deshpande S, et al. Efficacy of midodrine hydrochloride in neurocardiogenic syncope refractory to standard therapy. J Cardiovasc Electrophysiol 1997; 8: 42-6.
55. Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: A modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38: 1491-6.
56. Young TM, Mathias CJ. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: Multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry 2004; 75: 1737-41.
57. Ozcan C, Jahangir A, Friedman PA, Patel PJ, Munger TM, Rea RF, et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med 2001; 344: 1043-51.
58. Olshansky B, Poole JE, Johnson G, Anderson J, Hellkamp AS, Packer D, et al. Syncope predicts the outcome of cardiomyopathy patients: Analysis of the SCD-HeFT study. J Am Coll Cardiol 2008; 51: 1277-82.
59. Shechter A, Stewart WF, Silberstein SD, Lipton RB. Migraine and autonomic nervous system function: A population-based, case-control study. Neurology 2002; 58: 422-7.
60. Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2000; 55: 754-62.
61. Hadjipetrou P, Cox S, Piemonte T, Eisenhauer A. Percutaneous revascularization of atherosclerotic obstruction of aortic arch vessels. J Am Coll Cardiol 1999; 33: 1238-45.