1. Introduction
In Colombia, pineapple and flower crops play a significant role in the economy. In the first case, there are 17 departments that cultivated 25,764 hectares, with an average production of 30 ton/ha and with exports in 2017 of USD 31.1 million1. It is important to mention that 66% of the cut flowers that are exported come from the department of Cundinamarca, 32% from Antioquia, and the western center (Tolima, Bogotá, Boyacá, etc.) contributes with a 2%. According to ProColombia, this industry generated about USD 1,463 million in 2018, and for each cultivated hectare, 17 direct and indirect jobs in 60 Colombian municipalities were created. It has a business network of more than 400 companies. On the other hand, according to Asocolflores2, floriculture in Colombia generates more than 140 thousand formal jobs, from which about 65% of the workers in the sector are head-of-household mothers, and 40% of exports are certified "Florverde Sustainable Flowers".
Pineapple and flower crops are susceptible to various pests and diseases that limit their production, such is the case of the damage caused by phytoparasitic nematodes, hindering in most producing areas of the world3. There are 4,105 known species of phytoparasitic nematodes, which generate annual losses between 11 and 14%, equivalent to USD 80 billion per year4. Granulated organophosphates and liquid pyrethroids are used for their control; however, the emergence of resistance, export restrictions due to the use of chemicals and green stamps require to seek for strains of native microorganisms that can be efficiently produced in different crops5.
For several years, investigations have been carried out with the fungus Purpureocillium sp., Udea0106 strain, isolated from the soil in Colombia. Its pathogenicity on nematodes was previously demonstrated in vitro and in the field, applying the raw filtrate of the fungus, using both artisanal massive multiplication6 and a semi-artisan bioformulate where pathogenic activity was shown on the genera Meloidogyne spp. and Paratylenchus spp. in chrysanthemum crops7. Additionally, its pathogenicity has been efficiently demonstrated on symphilids8, and studies on genomics and transcriptomics are currently being developed to establish the taxonomic classification and the mechanisms of interaction with their hosts9.
The present research shows results about bioassays carried out on a laboratory scale, through a simple methodology that evaluates nematode populations directly on pineapple and flower soils, in a short period of time. The fungus used was produced on a small scale under bioreactor conditions in an agitated tank and the number of phytopathogenic nematodes decreased; for some, no previous records of biocontrol with fungi had been found.
2. Material and methods
2.1 Soil sampling
To carry out the bioassays, soil samples were taken from pineapple crops from two different geographical areas (Quindío and Cauca), and two flower crops (chrysanthemum) in Antioquia, that had previously recorded nematodes. For both crops, 4 to 6 sites were selected per farm and geographic region. Pineapple soil samples weighed 300 to 400 g, while 100 to 200 g of flower soil was collected. The collected samples were taken to the Fitobiol laboratory of the University of Antioquia, Medellin headquarters, for processing.
2.2 Mass multiplication in bioreactor
For the fungus mass multiplication, a 14-l agitated tank bioreactor was used, with an effective working volume of 10 l. Its dimensions were 0.16 m internal diameter, with 0.009 m baffles, a perforated aerator and a Rushton turbine-type agitator with a diameter of 0.048 m. Values of pH and dissolved oxygen (do) were recorded, agitation speed of 350 rpm and temperature of 27 °C for the continuous production of the fungus were determined. The culture medium used supplied the required sources of carbon, nitrogen and salts for the fungus development (unpublished data - personal communication Juan Diego Medina, October 2019), and after four days the fungus was harvested, with concentrations of 1x109 con/ml.
2.3. Bioassay setup
A suspension of 1x108 con/ml was prepared with the fungus obtained by fermentation and 10 ml of it were directly applied to each experimental unit, which consisted of polystyrene containers with lid (Type T1- Darnel®), containing 100 g of soil from each sample. Following the application, the soil and the fungus of each container were homogenized manually and incubated for 48 hours at 25 °C (+\- 5 °C); after this time, the nematodes were extracted using the sieve or extraction tray method9, to be subsequently concentrated in 20 ml of tap water.
Once the solutions with nematodes were obtained, each repetition was counted, by means of optical microscopy, at 4x and 10x. The variable to evaluate was the number of nematodes in 100 g of soil.
2.4. Experimental design
The evaluated treatments corresponded to the concentration of the fungus of 1x108 con/ml in each experimental unit. Containers with soil and sterile distilled water (SDW) were used as control. For each farm and in each crop, 4 to 6 repetitions were performed, corresponding to soils taken from the sites described in section 2.1. The bioassays were carried out at different times to determine any statistically significant differences between treatments and control; Kruskal-Wallis and Van der Waerden tests were completed to evaluate differences in the groups (P=0.05), using the R studio® Software and the Agricolae package.
2.5 Data transparency
Available data: All databases related to the statistical analysis of the tests performed, as well as the R software scripts.
Unavailable data: Those components of the culture medium related to where the fungus multiplied, given that they are part of a master's degree thesis and have not yet been published.
3. Results and discussion
The nematodes found in this study correspond to some of those registered by other authors in different crops of economic importance10, as well as to pineapple and chrysanthemum3)(11.
As it can be observed in the figures corresponding to crops of two different regions, for both pineapple and flowers (Figures 1, 2, 3 and 4), the genera of phytopathogenic nematodes vary even within the same crop. This depends on climatic conditions, soil types, forms of management, as well as the previous crops.
Due to variations in the population of these organisms, it is crucial to count with a biocontroller that can act on several genera of nematodes, in different soils and crops. Accordingly, it should be noted that, under the evaluated conditions, Purpureocillium sp. UdeA0106 strain decreased nematodes of the genera Meloidogyne spp., Helicotylenchus spp., Paratylenchus spp. and to a lesser extent Tylenchus spp.
In light of the results obtained, this fungus does not significantly decrease populations of Aphelenchoides spp. and Paratylenchus spp. in the evaluated crops. However, a greater number of studies in other crops must be performed before reaching conclusions, especially since the fungus-nematode interaction is influenced by plant type, as observed since the 1990s. Differences in the control have been recorded in the ratio of Meloidogyne spp. with the same genus of biocontroller fungus. This is due to the presence of different exudates emitted by plants to the soil that can influence the establishment and action of the fungus12.
It is important to highlight that biocontroller fungi recorded so far by other authors and those produced by commercial houses usually decrease the population of Meloidogyne spp13)(14)(15)(16, which was ratified in this study and agrees with Sánchez and others7. Their research reported than in flowers, the fungus UdeA0106 decreases the population of Meloidogyne spp. and, additionally, the genus Paratylenchus spp. when using a different methodology from that developed in this study. Nonetheless, the importance of this study is that no biocontroller fungi had been recorded yet for the genera Helicotylenchus spp. and Tylenchus spp., economically important nematodes in different crops17)(18. As can be identified in Figures 1 and 2, in pineapple crops, the genus Helicotylenchus spp. was decreased by almost a third, while Tylenchus spp. was decreased by more than 50%, when compared to their control.
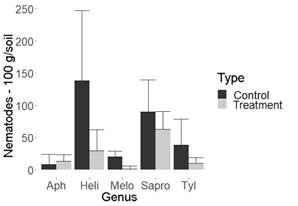
Figure 1: Nematode population in 100 g of pineapple soil. Montenegro (Quindio, Colombia). Aph= Aphelenchoides spp., Heli= Helicotylenchus spp., Melo=Meloidogyne spp., Sapro=Saprofitos, Tyl= Tylenchus spp.
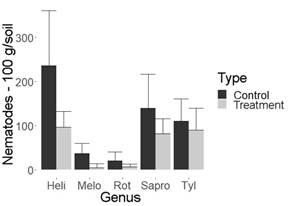
Figure 2: Nematode population in 100 g of pineapple soil. Miranda (Cauca, Colombia). Aph= Aphelenchoides spp., Heli= Helicotylenchus spp., Melo=Meloidogyne spp., Sapro=Saprofitos, Tyl= Tylenchus spp.
On the other hand, biocontroller fungi have been massively multiplied by solid fermentation for several years using organic substrates such as rice, barley or oats19; moreover, since 2008, a patent regulates liquid production of the fungus Pochonia chlamydosppora (current Metacordyceps chlamydospporia), which is also biocontroller of Meloidogyne spp.20, mainly for eggs. This could be considered in the future, concerning the medium used for the strain under study.
A desirable condition in the multiplication of fungi is to achieve the production of resistance structures through liquid fermentation. This is how some authors achieved the production of microsclerotia in the fungus P.lilacinum21, although this was not observed in the present study, under the evaluated conditions.
Despite the above, among the advantages of the production of this fungus in liquid medium by agitated tank is the increase in the concentration of conidia in a shorter time, compared to solid fermentation in rice substrate (personal communication Juan Diego Medina 2019, unpublished data). Likewise, Sharma and others12 pointed out how through this type of liquid fermentation some fungi achieve the production of metabolites that are dissolved in the medium. This has also been recorded by Sánchez in 201722, who demonstrated how in a liquid culture medium the UdeA0106 strain transcribes pathogenicity candidate genes, such as serine proteases, lipases and chitinases, which could explain its rapid action in just 48 hours of fungus-nematode contact in soil. This had already been demonstrated in Petri box7.
Despite the ability of the evaluated fungus to decrease the population of phytopathogenic nematodes, according to the results, no significant differences were observed between saprophytes with fungus treatment and its control in all cultures studied. This could be a desirable aspect from an ecological point of view (Figure 1, 2, 3 and 4, Table 1).
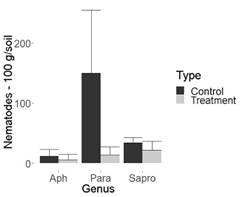
Figure 3: Nematode population in 100 g of chrysanthemum soil. Flores Esmeralda (La Ceja-Antioquia, Colombia). Aph= Aphelenchoides spp., Para=Paratylenchus spp., Sapro= Saprofitos
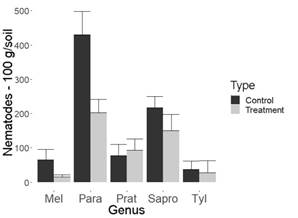
Figure 4: Nematode population in 100 g of chrysanthemum soil. Flores Miramonte (Marinilla-Antioquia, Colombia). Mel= Meloidogyne spp., Para=Paratylenchus spp., Prat= Pratylenchus spp., Tyl=Tylenchus spp.
In Table 1 it can be observed that in the same genus of nematodes there may or may not exist significant differences between treatment and control (highlighted in gray), while in other cases no significance is observed. The above mentioned could be explained by intraspecific differences within each nematode population in the evaluated crops, which could also indicate fungus specificity by some types of nematodes.
Table 1: Significance values according to Kruskal-Wallis. P=0.05
| Pineapple | Flowers | ||||
| Miranda | Montenegro | Miramonte | Esmeralda | ||
| Mel | 0,0010 | 0,0043 | 0,0371 | - | |
| Tyl | 0,4942 | 0,0321 | 0,2338 | - | |
| Para | - | - | 0,0209 | 0,0086 | |
| Praty | - | - | 0,3807 | - | |
| Helico | 0,0027 | 0,0198 | - | - | |
| Aph. | - | 0,2043 | - | 0,3556 | |
| Sapro | 0,0713 | 0,7838 | 0,0795 | 0,1461 | |
Mel= Meloidogyne spp., Tyl= Tylenchus spp., Para=Paratylenchus spp, Praty= Paratylenchus spp., Helico= Helicotylenchus spp., Aph= Aphelenchoides spp., Sapro= Saprofitos
4. Conclusions
The study revealed the possibility to accomplish rapid evaluations on the ability of a biocontroller fungus to decrease the nematode population through the polystyrene container technique used. Furthermore, it was demonstrated how this fungus can biocontrol genera such as Meloidogyne spp. and Paratylenchus spp., for which there are previous reports of biocontroller fungi. It also affects other genera of economic importance, such as Helicotylenchus spp. and Tylenchus spp., for which no reports of biocontrol with fungi had previously been found. The study also showed that the technique of fungus production in a fermented tank allows obtaining biocontroller fungi in a short period of time, and is efficient under laboratory conditions.















